New Findings: Hindlimb Unloading Causes Nucleocytoplasmic Ca2+ Overload and DNA Damage in Skeletal Muscle
- PMID: 37048150
- PMCID: PMC10093444
- DOI: 10.3390/cells12071077
New Findings: Hindlimb Unloading Causes Nucleocytoplasmic Ca2+ Overload and DNA Damage in Skeletal Muscle
Abstract
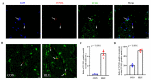
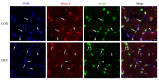
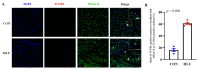
Disuse atrophy of skeletal muscle is associated with a severe imbalance in cellular Ca2+ homeostasis and marked increase in nuclear apoptosis. Nuclear Ca2+ is involved in the regulation of cellular Ca2+ homeostasis. However, it remains unclear whether nuclear Ca2+ levels change under skeletal muscle disuse conditions, and whether changes in nuclear Ca2+ levels are associated with nuclear apoptosis. In this study, changes in Ca2+ levels, Ca2+ transporters, and regulatory factors in the nucleus of hindlimb unloaded rat soleus muscle were examined to investigate the effects of disuse on nuclear Ca2+ homeostasis and apoptosis. Results showed that, after hindlimb unloading, the nuclear envelope Ca2+ levels ([Ca2+]NE) and nucleocytoplasmic Ca2+ levels ([Ca2+]NC) increased by 78% (p < 0.01) and 106% (p < 0.01), respectively. The levels of Ca2+-ATPase type 2 (Ca2+-ATPase2), Ryanodine receptor 1 (RyR1), Inositol 1,4,5-tetrakisphosphate receptor 1 (IP3R1), Cyclic ADP ribose hydrolase (CD38) and Inositol 1,4,5-tetrakisphosphate (IP3) increased by 470% (p < 0.001), 94% (p < 0.05), 170% (p < 0.001), 640% (p < 0.001) and 12% (p < 0.05), respectively, and the levels of Na+/Ca2+ exchanger 3 (NCX3), Ca2+/calmodulin dependent protein kinase II (CaMK II) and Protein kinase A (PKA) decreased by 54% (p < 0.001), 33% (p < 0.05) and 5% (p > 0.05), respectively. In addition, DNase X is mainly localized in the myonucleus and its activity is elevated after hindlimb unloading. Overall, our results suggest that enhanced Ca2+ uptake from cytoplasm is involved in the increase in [Ca2+]NE after hindlimb unloading. Moreover, the increase in [Ca2+]NC is attributed to increased Ca2+ release into nucleocytoplasm and weakened Ca2+ uptake from nucleocytoplasm. DNase X is activated due to elevated [Ca2+]NC, leading to DNA fragmentation in myonucleus, ultimately initiating myonuclear apoptosis. Nucleocytoplasmic Ca2+ overload may contribute to the increased incidence of myonuclear apoptosis in disused skeletal muscle.
Keywords: hindlimb unloading; nuclear Ca2+ regulation; nuclear apoptosis; skeletal muscle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Dietary astaxanthin supplementation attenuates disuse-induced muscle atrophy and myonuclear apoptosis in the rat soleus muscle.J Physiol Sci. 2017 Jan;67(1):181-190. doi: 10.1007/s12576-016-0453-4. Epub 2016 Apr 27. J Physiol Sci. 2017. PMID: 27117878 Free PMC article.
-
Loss of melusin is a novel, neuronal NO synthase/FoxO3-independent master switch of unloading-induced muscle atrophy.J Cachexia Sarcopenia Muscle. 2020 Jun;11(3):802-819. doi: 10.1002/jcsm.12546. Epub 2020 Mar 10. J Cachexia Sarcopenia Muscle. 2020. PMID: 32154658 Free PMC article.
-
Metformin Attenuates Slow-to-Fast Fiber Shift and Proteolysis Markers Increase in Rat Soleus after 7 Days of Rat Hindlimb Unloading.Int J Mol Sci. 2022 Dec 28;24(1):503. doi: 10.3390/ijms24010503. Int J Mol Sci. 2022. PMID: 36613942 Free PMC article.
-
Gravitational unloading effects on muscle fiber size, phenotype and myonuclear number.Adv Space Res. 2002;30(4):777-81. doi: 10.1016/s0273-1177(02)00395-2. Adv Space Res. 2002. PMID: 12530363 Review.
-
Various jobs of proteolytic enzymes in skeletal muscle during unloading: facts and speculations.J Biomed Biotechnol. 2012;2012:493618. doi: 10.1155/2012/493618. Epub 2012 Feb 8. J Biomed Biotechnol. 2012. PMID: 22496611 Free PMC article. Review.
Cited by
-
Role of PI3 Kinases in Cell Signaling and Soleus Muscle Atrophy During Three Days of Unloading.Int J Mol Sci. 2025 Jan 6;26(1):414. doi: 10.3390/ijms26010414. Int J Mol Sci. 2025. PMID: 39796270 Free PMC article.
References
-
- Wu X., Gao Y.F., Zhao X.H., Cui J.H. Effects of tetramethylpyrazine on nitric oxide synthase activity and calcium ion concentration of skeletal muscle in hindlimb unloading rats. Zhonghua Yi Xue Za Zhi. 2012;92:2075–2077. - PubMed
-
- Ingalls C.P., Wenke J.C., Armstrong R.B. Time course changes in [Ca2+]i, force, and protein content in hindlimb-suspended mouse soleus muscles. Aviat. Space Environ. Med. 2001;72:471–476. - PubMed
-
- Tomiya S., Tamura Y., Kouzaki K., Kotani T., Wakabayashi Y., Noda M., Nakazato K. Cast immobilization of hindlimb upregulates sarcolipin expression in atrophied skeletal muscles and increases thermogenesis in C57BL/6J mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019;317:649–661. doi: 10.1152/ajpregu.00118.2019. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

