High Mobility Group Box 1 (HMGB1): Potential Target in Sepsis-Associated Encephalopathy
- PMID: 37048161
- PMCID: PMC10093266
- DOI: 10.3390/cells12071088
High Mobility Group Box 1 (HMGB1): Potential Target in Sepsis-Associated Encephalopathy
Abstract
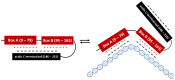
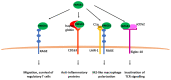
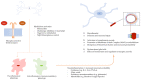
Sepsis-associated encephalopathy (SAE) remains a challenge for intensivists that is exacerbated by lack of an effective diagnostic tool and an unambiguous definition to properly identify SAE patients. Risk factors for SAE development include age, genetic factors as well as pre-existing neuropsychiatric conditions. Sepsis due to certain infection sites/origins might be more prone to encephalopathy development than other cases. Currently, ICU management of SAE is mainly based on non-pharmacological support. Pre-clinical studies have described the role of the alarmin high mobility group box 1 (HMGB1) in the complex pathogenesis of SAE. Although there are limited data available about the role of HMGB1 in neuroinflammation following sepsis, it has been implicated in other neurologic disorders, where its translocation from the nucleus to the extracellular space has been found to trigger neuroinflammatory reactions and disrupt the blood-brain barrier. Negating the inflammatory cascade, by targeting HMGB1, may be a strategy to complement non-pharmacologic interventions directed against encephalopathy. This review describes inflammatory cascades implicating HMGB1 and strategies for its use to mitigate sepsis-induced encephalopathy.
Keywords: HMGB1; high mobility group box 1; sepsis; sepsis-associated encephalopathy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

