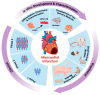
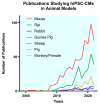
Preclinical Large Animal Porcine Models for Cardiac Regeneration and Its Clinical Translation: Role of hiPSC-Derived Cardiomyocytes
- PMID: 37048163
- PMCID: PMC10093073
- DOI: 10.3390/cells12071090
Preclinical Large Animal Porcine Models for Cardiac Regeneration and Its Clinical Translation: Role of hiPSC-Derived Cardiomyocytes
Abstract
Myocardial Infarction (MI) occurs due to a blockage in the coronary artery resulting in ischemia and necrosis of cardiomyocytes in the left ventricular heart muscle. The dying cardiac tissue is replaced with fibrous scar tissue, causing a decrease in myocardial contractility and thus affecting the functional capacity of the myocardium. Treatments, such as stent placements, cardiac bypasses, or transplants are beneficial but with many limitations, and may decrease the overall life expectancy due to related complications. In recent years, with the advent of human induced pluripotent stem cells (hiPSCs), newer avenues using cell-based approaches for the treatment of MI have emerged as a potential for cardiac regeneration. While hiPSCs and their derived differentiated cells are promising candidates, their translatability for clinical applications has been hindered due to poor preclinical reproducibility. Various preclinical animal models for MI, ranging from mice to non-human primates, have been adopted in cardiovascular research to mimic MI in humans. Therefore, a comprehensive literature review was essential to elucidate the factors affecting the reproducibility and translatability of large animal models. In this review article, we have discussed different animal models available for studying stem-cell transplantation in cardiovascular applications, mainly focusing on the highly translatable porcine MI model.
Keywords: cell transplantation; hiPSC-CMs; large animal model; myocardial infarction; porcine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Thymosin β4 increases cardiac cell proliferation, cell engraftment, and the reparative potency of human induced-pluripotent stem cell-derived cardiomyocytes in a porcine model of acute myocardial infarction.Theranostics. 2021 Jun 26;11(16):7879-7895. doi: 10.7150/thno.56757. eCollection 2021. Theranostics. 2021. PMID: 34335970 Free PMC article.
-
Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells.Cell Stem Cell. 2014 Dec 4;15(6):750-61. doi: 10.1016/j.stem.2014.11.009. Cell Stem Cell. 2014. PMID: 25479750 Free PMC article.
-
Comparison of human induced pluripotent stem-cell derived cardiomyocytes with human mesenchymal stem cells following acute myocardial infarction.PLoS One. 2014 Dec 31;9(12):e116281. doi: 10.1371/journal.pone.0116281. eCollection 2014. PLoS One. 2014. PMID: 25551230 Free PMC article.
-
Maturation of pluripotent stem cell-derived cardiomyocytes: limitations and challenges from metabolic aspects.Stem Cell Res Ther. 2024 Oct 8;15(1):354. doi: 10.1186/s13287-024-03961-4. Stem Cell Res Ther. 2024. PMID: 39380099 Free PMC article. Review.
-
Robust Cardiac Regeneration: Fulfilling the Promise of Cardiac Cell Therapy.Clin Ther. 2020 Oct;42(10):1857-1879. doi: 10.1016/j.clinthera.2020.08.008. Epub 2020 Sep 14. Clin Ther. 2020. PMID: 32943195 Review.
Cited by
-
Delivery of Stem Cells and BMP-2 With Functionalized Self-Assembling Peptide Enhances Regeneration of Infarcted Myocardium.Stem Cell Rev Rep. 2024 Aug;20(6):1540-1554. doi: 10.1007/s12015-024-10721-7. Epub 2024 Apr 24. Stem Cell Rev Rep. 2024. PMID: 38656478
-
Effect of metabolically divergent pig breeds and tissues on mesenchymal stem cell expression patterns during adipogenesis.BMC Genomics. 2024 Apr 25;25(1):407. doi: 10.1186/s12864-024-10308-z. BMC Genomics. 2024. PMID: 38664635 Free PMC article.
-
Fabrication of heart tubes from iPSC derived cardiomyocytes and human fibrinogen by rotating mold technology.Sci Rep. 2024 Jun 7;14(1):13174. doi: 10.1038/s41598-024-64022-7. Sci Rep. 2024. PMID: 38849457 Free PMC article.
-
Editorial: Methods in cardiovascular biologics and regenerative medicine.Front Cardiovasc Med. 2024 Sep 13;11:1477927. doi: 10.3389/fcvm.2024.1477927. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39350966 Free PMC article. No abstract available.
-
Klotho/FGF23 Axis Regulates Cardiomyocyte Apoptosis and Cytokine Release through ERK/MAPK Pathway.Cardiovasc Toxicol. 2023 Oct;23(9-10):317-328. doi: 10.1007/s12012-023-09805-6. Epub 2023 Sep 13. Cardiovasc Toxicol. 2023. PMID: 37704925
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical