Glucagon Promotes Gluconeogenesis through the GCGR/PKA/CREB/PGC-1α Pathway in Hepatocytes of the Japanese Flounder Paralichthys olivaceus
- PMID: 37048171
- PMCID: PMC10093564
- DOI: 10.3390/cells12071098
Glucagon Promotes Gluconeogenesis through the GCGR/PKA/CREB/PGC-1α Pathway in Hepatocytes of the Japanese Flounder Paralichthys olivaceus
Abstract
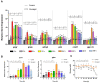
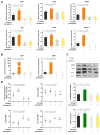
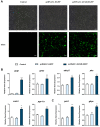
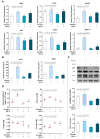
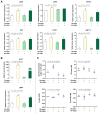
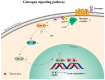
In order to investigate the mechanism of glucagon regulation of gluconeogenesis, primary hepatocytes of the Japanese flounder (Paralichthys olivaceus) were incubated with synthesized glucagon, and methods based on inhibitors and gene overexpression were employed. The results indicated that glucagon promoted glucose production and increased the mRNA levels of glucagon receptor (gcgr), guanine nucleotide-binding protein Gs α subunit (gnas), adenylate cyclase 2 (adcy2), protein kinase A (pka), cAMP response element-binding protein 1 (creb1), peroxisome proliferator-activated receptor-γ coactivator 1α (pgc-1α), phosphoenolpyruvate carboxykinase 1 (pck1), and glucose-6-phosphatase (g6pc) in the hepatocytes. An inhibitor of GCGR decreased the mRNA expression of gcgr, gnas, adcy2, pka, creb1, pgc-1α, pck1, g6pc, the protein expression of phosphorylated CREB and PGC-1α, and glucose production. The overexpression of gcgr caused the opposite results. An inhibitor of PKA decreased the mRNA expression of pgc-1α, pck1, g6pc, the protein expression of phosphorylated-CREB, and glucose production in hepatocytes. A CREB-targeted inhibitor significantly decreased the stimulation by glucagon of the mRNA expression of creb1, pgc-1α, and gluconeogenic genes, and glucose production decreased accordingly. After incubating the hepatocytes with an inhibitor of PGC-1α, the glucagon-activated mRNA expression of pck1 and g6pc was significantly down-regulated. Together, these results demonstrate that glucagon promotes gluconeogenesis through the GCGR/PKA/CREB/PGC-1α pathway in the Japanese flounder.
Keywords: Paralichthys olivaceus; glucagon; gluconeogenesis; glucose; signaling pathway.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
CREB-upregulated lncRNA MEG3 promotes hepatic gluconeogenesis by regulating miR-302a-3p-CRTC2 axis.J Cell Biochem. 2019 Mar;120(3):4192-4202. doi: 10.1002/jcb.27706. Epub 2018 Sep 27. J Cell Biochem. 2019. PMID: 30260029
-
Decreased Gluconeogenesis in the Absence of Cystathionine Gamma-Lyase and the Underlying Mechanisms.Antioxid Redox Signal. 2016 Jan 20;24(3):129-40. doi: 10.1089/ars.2015.6369. Epub 2015 Sep 24. Antioxid Redox Signal. 2016. PMID: 26401978 Free PMC article.
-
Regulation of Hepatic Gluconeogenesis by Nuclear Receptor Coactivator 6.Mol Cells. 2022 Apr 30;45(4):180-192. doi: 10.14348/molcells.2022.2222. Mol Cells. 2022. PMID: 35258009 Free PMC article.
-
Exploring the signal-dependent transcriptional regulation involved in the liver pathology of type 2 diabetes.Diabetol Int. 2022 Dec 3;14(1):15-20. doi: 10.1007/s13340-022-00610-0. eCollection 2023 Jan. Diabetol Int. 2022. PMID: 36636166 Free PMC article. Review.
-
Gastrointestinal: Pancreatic NETs with GCGR heterozygous mutation: Mahvash disease.J Gastroenterol Hepatol. 2023 Aug;38(8):1243. doi: 10.1111/jgh.16104. Epub 2023 Jan 25. J Gastroenterol Hepatol. 2023. PMID: 36698259 Review. No abstract available.
Cited by
-
The Neurometabolic Function of the Dopamine-Aminotransferase System.Metabolites. 2025 Jan 6;15(1):21. doi: 10.3390/metabo15010021. Metabolites. 2025. PMID: 39852364 Free PMC article. Review.
-
Small molecules targeting selective PCK1 and PGC-1α lysine acetylation cause anti-diabetic action through increased lactate oxidation.Cell Chem Biol. 2024 Oct 17;31(10):1772-1786.e5. doi: 10.1016/j.chembiol.2024.09.001. Epub 2024 Sep 27. Cell Chem Biol. 2024. PMID: 39341205
References
-
- Park M.K. Glucagon. In: Takei Y., Ando H., Tsutsui K., editors. Handbook of Hormones. Academic Press; Oxford, UK: 2016. pp. 129–131.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

