Proposal and Verification of the Theory of Layer-by-Layer Elimination of Biofilm in Listeria monocytogenes
- PMID: 37048183
- PMCID: PMC10093742
- DOI: 10.3390/foods12071361
Proposal and Verification of the Theory of Layer-by-Layer Elimination of Biofilm in Listeria monocytogenes
Abstract
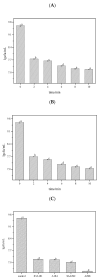
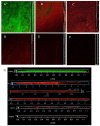
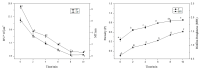
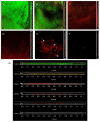
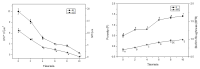
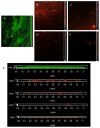
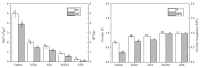
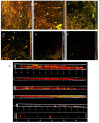
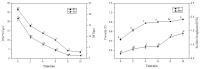
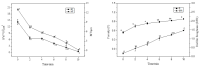
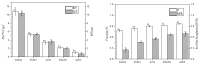
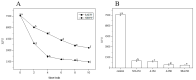
Biofilms are microbial communities that represent a high abundance of microbial life forms on Earth. Within biofilms, structural changes during clearance processes occur in three spatial and temporal dimensions; therefore, microscopy and quantitative image analysis are essential in elucidating their function. Here, we present confocal laser scanning microscopy (CLSM) in conjunction with ISA-2 software analysis for the automated and high-throughput quantification, analysis, and visualisation of biofilm interiors and overall biofilm properties in three spatial and temporal dimensions. This paper discusses the removal process of Listeria monocytogenes (LM) biofilms using slightly acidic electrolytic water, non-electrolytic hypochlorite water, and alternating the use of strongly acidic and strongly alkaline electrolytic water. The results show that the biofilm gradually thins and gutters from the initial viscous dense and thick morphology under the action of either biocide. This process is consistent with first-level kinetics. After CLSM filming to observe the biofilm structure, analysis software was used to process and quantify the biovolume, average biofilm thickness, biofilm roughness and other indicators; fluorescence enzyme markers were used to verify the remaining amount of extracellular nucleic acid. In this study, we proposed and validated the theory of layer-by-layer elimination of LM biofilm.
Keywords: Listeria monocytogenes; biofilms; confocal laser scanning microscopy; electrolysed water; layer-by-layer elimination.
Conflict of interest statement
The authors declare no competing financial or non-financial Interest.
Figures


Similar articles
-
Characterization of Binary Biofilms of Listeria monocytogenes and Lactobacillus and Their Response to Chlorine Treatment.Front Microbiol. 2021 Jul 14;12:638933. doi: 10.3389/fmicb.2021.638933. eCollection 2021. Front Microbiol. 2021. PMID: 34335486 Free PMC article.
-
Characterization of Listeria monocytogenes biofilm formation kinetics and biofilm transfer to cantaloupe surfaces.Food Res Int. 2022 Nov;161:111839. doi: 10.1016/j.foodres.2022.111839. Epub 2022 Aug 27. Food Res Int. 2022. PMID: 36192897
-
Mechanism of acid and alkali electrolyzed water on the elimination of Listeria monocytogenes biofilm based on proteomic analysis.J Proteomics. 2023 Aug 30;286:104952. doi: 10.1016/j.jprot.2023.104952. Epub 2023 Jun 29. J Proteomics. 2023. PMID: 37390895
-
Microbial communities and their interactions in biofilm systems: an overview.Water Sci Technol. 2004;49(11-12):327-36. Water Sci Technol. 2004. PMID: 15303758 Review.
-
Tools of the Trade: Image Analysis Programs for Confocal Laser-Scanning Microscopy Studies of Biofilms and Considerations for Their Use by Experimental Researchers.ACS Omega. 2023 May 26;8(23):20163-20177. doi: 10.1021/acsomega.2c07255. eCollection 2023 Jun 13. ACS Omega. 2023. PMID: 37332792 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

