Characterization of the Biofilms Formed by Histamine-Producing Lentilactobacillus parabuchneri Strains in the Dairy Environment
- PMID: 37048324
- PMCID: PMC10093819
- DOI: 10.3390/foods12071503
Characterization of the Biofilms Formed by Histamine-Producing Lentilactobacillus parabuchneri Strains in the Dairy Environment
Abstract
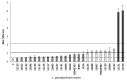
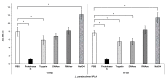
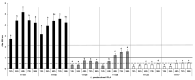
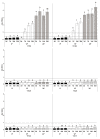
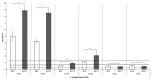
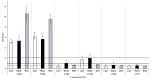
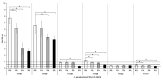
Lentilactobacillus parabuchneri, a lactic acid bacterium, is largely responsible for the production and accumulation of histamine, a toxic biogenic amine, in cheese. L. parabuchneri strains can form biofilms on the surface of industry equipment. Since they are resistant to cleaning and disinfection, they may act as reservoirs of histamine-producing contaminants in cheese. The aim of this study was to investigate the biofilm-producing capacity of L. parabuchneri strains. Using the crystal violet technique, the strains were first categorized as weak, moderate or strong biofilm producers. Analysis of their biofilm matrices revealed them to be mainly composed of proteins. Two strains of each category were then selected to analyze the influence on the biofilm-forming capacity of temperature, pH, carbon source, NaCl concentration and surface material (i.e., focusing on those used in the dairy industry). In general, low temperature (8 °C), high NaCl concentrations (2-3% w/v) and neutral pH (pH 6) prevented biofilm formation. All strains were found to adhere easily to beech wood. These findings increase knowledge of the biofilm-forming capacity of histamine-producing L. parabuchneri strains and how their formation may be prevented for improving food safety.
Keywords: Lentilactobacillus parabuchneri; biofilms; biogenic amines; histamine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The gene cluster associated with strong biofilm-formation capacity by histamine-producing Lentilactobacillus parabuchneri encodes a sortase-mediated pilus and is located on a plasmid.Food Res Int. 2024 Jan;175:113777. doi: 10.1016/j.foodres.2023.113777. Epub 2023 Nov 26. Food Res Int. 2024. PMID: 38129064
-
Rapid discrimination of Lentilactobacillus parabuchneri biofilms via in situ infrared spectroscopy.Spectrochim Acta A Mol Biomol Spectrosc. 2024 Jan 5;304:123391. doi: 10.1016/j.saa.2023.123391. Epub 2023 Sep 9. Spectrochim Acta A Mol Biomol Spectrosc. 2024. PMID: 37714102
-
Biofilm-Forming Capacity in Biogenic Amine-Producing Bacteria Isolated from Dairy Products.Front Microbiol. 2016 May 12;7:591. doi: 10.3389/fmicb.2016.00591. eCollection 2016. Front Microbiol. 2016. PMID: 27242675 Free PMC article.
-
Comparative Genomics of Lentilactobacillus parabuchneri isolated from dairy, KEM complex, Makgeolli, and Saliva Microbiomes.BMC Genomics. 2022 Dec 5;23(1):803. doi: 10.1186/s12864-022-09053-y. BMC Genomics. 2022. PMID: 36471243 Free PMC article.
-
Histamine accumulation in dairy products: Microbial causes, techniques for the detection of histamine-producing microbiota, and potential solutions.Compr Rev Food Sci Food Saf. 2021 Mar;20(2):1481-1523. doi: 10.1111/1541-4337.12704. Epub 2021 Jan 27. Compr Rev Food Sci Food Saf. 2021. PMID: 33506573 Review.
Cited by
-
Development of super nanoantimicrobials combining AgCl, tetracycline and benzalkonium chloride.Discov Nano. 2024 Jun 11;19(1):100. doi: 10.1186/s11671-024-04043-3. Discov Nano. 2024. PMID: 38861141 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

