The Stromal Vascular Fraction from Canine Adipose Tissue Contains Mesenchymal Stromal Cell Subpopulations That Show Time-Dependent Adhesion to Cell Culture Plastic Vessels
- PMID: 37048431
- PMCID: PMC10093060
- DOI: 10.3390/ani13071175
The Stromal Vascular Fraction from Canine Adipose Tissue Contains Mesenchymal Stromal Cell Subpopulations That Show Time-Dependent Adhesion to Cell Culture Plastic Vessels
Abstract
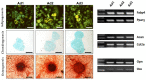
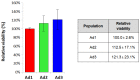
Adipose-derived mesenchymal stromal cells (MSCs) are extensively studied in both human and veterinary medicine. Their isolation is usually performed by collagenase digestion followed by filtration and removal of nonadherent tissue remnants 48 h after seeding. We observed that waste tissue fragments contain cells that adhere belatedly to the plastic. We aimed to investigate their basic properties to speculate on the possible existence of MSC subpopulations. Adipose tissue from three dogs was enzymatically digested. Three cell populations that adhered to the culture plastic 48, 96, and 144 h after seeding were obtained. After expansion, they were analyzed by flow cytometry for MSC-positive (CD90, CD44, and CD29) and -negative (CD14, MHCII, and CD45) markers as well as for endothelial, pericyte, and smooth muscle cell markers (CD31, CD146, and alpha-SMA). Furthermore, cells were assessed for viability, doubling time, and trilineage differentiation ability. No significant differences were found between the three subpopulations. As a result, this procedure has proven to be a valuable method for dramatically improving MSCs yield. As a consequence of cell recovery optimization, the amount of tissue harvested could be reduced, and the time required to obtain sufficient cells for clinical applications could be shortened. Further studies are needed to uncover possible different functional properties.
Keywords: adipose tissue; cell culture; dog; immunophenotype; mesenchymal stromal/stem cells; stromal vascular fraction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Stromal vascular progenitors in adult human adipose tissue.Cytometry A. 2010 Jan;77(1):22-30. doi: 10.1002/cyto.a.20813. Cytometry A. 2010. PMID: 19852056 Free PMC article.
-
Serum-free human MSC medium supports consistency in human but not in equine adipose-derived multipotent mesenchymal stromal cell culture.Cytometry A. 2018 Jan;93(1):60-72. doi: 10.1002/cyto.a.23240. Epub 2017 Sep 19. Cytometry A. 2018. PMID: 28926198
-
Characterization of vasculogenic potential of human adipose-derived endothelial cells in a three-dimensional vascularized skin substitute.Pediatr Surg Int. 2016 Jan;32(1):17-27. doi: 10.1007/s00383-015-3808-7. Epub 2015 Nov 30. Pediatr Surg Int. 2016. PMID: 26621500
-
Microfluidic Separation of Canine Adipose-Derived Mesenchymal Stromal Cells.Tissue Eng Part C Methods. 2021 Aug;27(8):445-461. doi: 10.1089/ten.TEC.2021.0082. Tissue Eng Part C Methods. 2021. PMID: 34155926
-
Defining adipose tissue-derived stem cells in tissue and in culture.Histol Histopathol. 2010 Jun;25(6):807-15. doi: 10.14670/HH-25.807. Histol Histopathol. 2010. PMID: 20376787 Review.
Cited by
-
Evaluation of a Novel Mechanical Device for the Production of Microfragmented Adipose Tissue for Veterinary Regenerative Medicine: A Proof-of-Concept.Int J Mol Sci. 2024 Nov 4;25(21):11854. doi: 10.3390/ijms252111854. Int J Mol Sci. 2024. PMID: 39519405 Free PMC article.
References
-
- Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F.C., Krause D.S., Deans R.J., Keating A., Prockop D.J., Horwitz E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

