Mortality Causes in Captive Cantabrian capercaillie (Tetrao urogallus cantabricus) in Spain
- PMID: 37048511
- PMCID: PMC10093503
- DOI: 10.3390/ani13071255
Mortality Causes in Captive Cantabrian capercaillie (Tetrao urogallus cantabricus) in Spain
Abstract
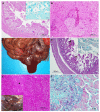
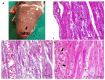
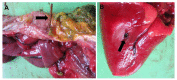
The Cantabrian capercaillie (Tetrao urogallus cantabricus) is one of the most severely threatened subspecies of capercaillie. Its current population range is restricted to a small area of the Cantabrian Mountains (northwestern Spain), with only around 200 individuals remaining. As part of the national strategy for the conservation of the subspecies, the Cantabrian capercaillie Captive Breeding Center of Sobrescobio opened in 2009. Here, we use the information provided by the necropsies performed in this facility on 29 individuals (11 males, 13 females and 5 undetermined; 16 chicks and 13 adults) in order to describe the main mortality causes of captive-bred Cantabrian capercaillies. After necropsy, tissue samples were taken for evaluation using standard methods in histology and microbiology. The majority of the captive animals (18/29, 62.07%) died due to infectious diseases, mainly due to Escherichia coli, Clostridium perfringens, or Aspergillus fumigatus infection. The remaining 11 animals died due to stress-related processes (i.e., rupture of the heart apex and cardiomyopathy or neurogenic shock) (8/29, 27.59%), duodenal obstruction and coelomitis (1/29, 3.45%), perforation of the proventriculus and heart with a briar branch (1/29, 3.45%) or euthanasia due to a valgus leg deformity that prevented proper animal welfare (1/29, 3.45%). Young animals (i.e., younger than 2 months) died mainly due to infectious diseases (14/16, 87.5%), while stress-related causes were responsible for most adult deaths (7/13, 53.85%). We additionally report that two free-ranging adult males died due to exertional myopathy. This study provides relevant information for reducing mortality in captive capercaillies and improving both living conditions in captivity and the adaptation of these animals to the wild.
Keywords: Cantabrian capercaillie; Tetrao urogallus cantabricus; aspergillosis; bacterial diseases; captivity; mortality causes; myopathy; pathology; valgus leg deformity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Use of native chicken breeds (Gallus gallus domesticus) for the development of suitable methods of Cantabrian capercaillie (Tetrao urogallus cantabricus) semen cryopreservation.Vet Med Sci. 2022 May;8(3):1311-1318. doi: 10.1002/vms3.742. Epub 2022 Apr 14. Vet Med Sci. 2022. PMID: 35419978 Free PMC article.
-
Parasites of the endangered Cantabrian capercaillie (Tetrao urogallus cantabricus): correlates with host abundance and lek site characteristics.Parasitol Res. 2008 Aug;103(3):709-12. doi: 10.1007/s00436-008-1015-3. Epub 2008 May 23. Parasitol Res. 2008. PMID: 18498000
-
The Cantabrian capercaillie: A population on the edge.Sci Total Environ. 2022 May 15;821:153523. doi: 10.1016/j.scitotenv.2022.153523. Epub 2022 Jan 29. Sci Total Environ. 2022. PMID: 35104529
-
Taxonomic inflation as a conservation trap for inbred populations.Evol Appl. 2024 May 8;17(5):e13677. doi: 10.1111/eva.13677. eCollection 2024 May. Evol Appl. 2024. PMID: 38721591 Free PMC article.
-
Sedation of Wild Pyrenean Capercaillie (Tetrao urogallus aquitanicus) Using Intramuscular Midazolam.Animals (Basel). 2022 Jul 11;12(14):1773. doi: 10.3390/ani12141773. Animals (Basel). 2022. PMID: 35883318 Free PMC article.
Cited by
-
First detection of herpesvirus and hemosporidians in the endangered Pyrenean Capercaillie (Tetrao urogallus aquitanicus).Sci Rep. 2023 Dec 11;13(1):21936. doi: 10.1038/s41598-023-48123-3. Sci Rep. 2023. PMID: 38081895 Free PMC article.
References
-
- Convention on Biological Diversity . Convention on Biological Diversity: Text and Annexes. Secretariat of the Convention on Biological Diversity; Montreal, QC, Canada: 1992. pp. 1–34.
-
- Maxted N., editor. Encyclopedia of Biodiversity. 2nd ed. Elsevier; Amsterdam, The Netherlands: 2013. In situ, ex situ conservation; pp. 313–323.
-
- Wakchaure R., Ganguly S. Captive breeding in endangered wildlife: A review. J. Biol. Sci. Opi. 2016;4:186. doi: 10.7897/2321-6328.04544. - DOI
-
- Snyder N.F., Derrickson S.R., Beissinger S.R., Wiley J.W., Smith T.B., Toone W.D., Miller B. Limitations of captive breeding in endangered species recovery. Conserv. Biol. 1996;10:338–348. doi: 10.1046/j.1523-1739.1996.10020338.x. - DOI
-
- Vargas A., Anderson S.H. Growth and physical development of captive-raised black-footed ferrets (Mustela nigripes) Am. Midl. Nat. 1996;135:43–52. doi: 10.2307/2426870. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

