Plasma Levels of Metalloproteinase 3 (MMP-3) and Metalloproteinase 7 (MMP-7) as New Candidates for Tumor Biomarkers in Diagnostic of Breast Cancer Patients
- PMID: 37048701
- PMCID: PMC10094779
- DOI: 10.3390/jcm12072618
Plasma Levels of Metalloproteinase 3 (MMP-3) and Metalloproteinase 7 (MMP-7) as New Candidates for Tumor Biomarkers in Diagnostic of Breast Cancer Patients
Abstract
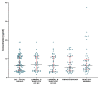
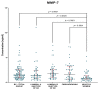
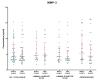
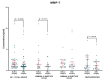
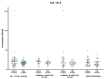
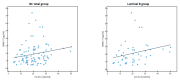
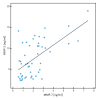
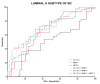
Matrix metalloproteinases (MMPs) are a group of enzymes that mediate both physiological and pathological processes such as carcinogenesis. The role of matrix metalloproteinase-3 (MMP-3) and (MMP-7) in the pathogenesis of breast cancer (BC) has been demonstrated, suggesting that they may be considered as potential markers of this condition. The aim of this study was to assess plasma concentrations and diagnostic utility of MMP-3 and MMP-7 in 100 patients with early-stage breast cancer with Luminal A subtype or Luminal B HER-negative subtype, before and after surgical treatment, and in the following control groups: patients with a benign tumor (fibroadenoma) and healthy subjects. The concentrations of MMP-3 and MMP-7 were referenced to the levels of the widely recognized marker for BC diagnosis CA 15-3. MMP-3 and MMP-7 was measured by ELISA method and CA 15-3 by CMIA. Plasma levels of MMP-7 were significantly higher in Luminal A and Luminal B HER2-negative subtype breast cancer patients as compared to the healthy group. MMP-7 demonstrated comparable but mostly higher to CA 15-3 or MMP-3 values of diagnostic sensitivity, specificity, positive and negative predictive values and AUC (0.6888 for Luminal A subtype; 0.7612 for Luminal B HER2-negative; 0.7250 for BC total group, respectively) in the groups tested. The combined use of the tested parameters resulted in a further increase in diagnostic criteria and AUC. These results suggest the usefulness of combining MMP-7 with CA 15-3 in the diagnostics of breast cancer, especially in Luminal B HER2-negative subtypes patients, as a new candidate for tumor markers.
Keywords: breast cancer; ca 15-3; fibroadenoma; luminal a subtype; luminal b her2-negative subtype; mmp-3; mmp-7.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Plasma Levels and Diagnostic Utility of M-CSF, MMP-2 and its Inhibitor TIMP-2 in the Diagnostics of Breast Cancer Patients.Clin Lab. 2016 Sep 1;62(9):1661-1669. doi: 10.7754/Clin.Lab.2016.160118. Clin Lab. 2016. PMID: 28164586
-
Plasma Levels of CXC Motif Chemokine 1 (CXCL1) and Chemokine 8 (CXCL8) as Diagnostic Biomarkers in Luminal A and B Breast Cancer.J Clin Med. 2022 Nov 12;11(22):6694. doi: 10.3390/jcm11226694. J Clin Med. 2022. PMID: 36431173 Free PMC article.
-
Plasma Levels and Diagnostic Utility of Macrophage Colony-Stimulating Factor, Matrix Metalloproteinase-9, and Tissue Inhibitor of Metalloproteinases-1 as New Biomarkers of Breast Cancer.Ann Lab Med. 2016 May;36(3):223-9. doi: 10.3343/alm.2016.36.3.223. Ann Lab Med. 2016. PMID: 26915610 Free PMC article.
-
CXCL12 and CXCR4 as Potential Early Biomarkers for Luminal A and Luminal B Subtypes of Breast Cancer.Cancer Manag Res. 2023 Jul 4;15:573-589. doi: 10.2147/CMAR.S416382. eCollection 2023. Cancer Manag Res. 2023. PMID: 37426394 Free PMC article.
-
Is There a Benefit of HER2-Positive Breast Cancer Subtype Determination in Clinical Practice?Klin Onkol. 2019 Winter;32(1):25-30. doi: 10.14735/amko2019. Klin Onkol. 2019. PMID: 30764626 Review. English.
Cited by
-
Diagnostic Utility of Selected Matrix Metalloproteinases (MMP-2, MMP-3, MMP-11, MMP-26), HE4, CA125 and ROMA Algorithm in Diagnosis of Ovarian Cancer.Int J Mol Sci. 2024 Jun 6;25(11):6265. doi: 10.3390/ijms25116265. Int J Mol Sci. 2024. PMID: 38892452 Free PMC article.
-
Plasma Concentrations of Matrilysins (MMP-7, MMP-26) and Stromelysins (MMP-3, MMP-10) as Diagnostic Biomarkers in High-Grade Serous Ovarian Cancer Patients.Int J Mol Sci. 2025 Jun 13;26(12):5661. doi: 10.3390/ijms26125661. Int J Mol Sci. 2025. PMID: 40565125 Free PMC article.
-
CXC ELR-Positive Chemokines as Diagnostic and Prognostic Markers for Breast Cancer Patients.Cancers (Basel). 2023 Jun 8;15(12):3118. doi: 10.3390/cancers15123118. Cancers (Basel). 2023. PMID: 37370728 Free PMC article. Review.
References
-
- Jones T., Duquette D., Underhill M., Ming C., Mendelsohn-Victor K.E., Anderson B., Milliron K.J., Copeland G., Janz N.K., Northouse L.L., et al. Surveillance for cancer recurrence in long-term young breast cancer survivors randomly selected from a statewide cancer registry. Breast Cancer Res. Treat. 2018;169:41–152. doi: 10.1007/s10549-018-4674-5. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

