Plasma-Polymerised Antibacterial Coating of Ovine Tendon Collagen Type I (OTC) Crosslinked with Genipin (GNP) and Dehydrothermal-Crosslinked (DHT) as a Cutaneous Substitute for Wound Healing
- PMID: 37049037
- PMCID: PMC10096142
- DOI: 10.3390/ma16072739
Plasma-Polymerised Antibacterial Coating of Ovine Tendon Collagen Type I (OTC) Crosslinked with Genipin (GNP) and Dehydrothermal-Crosslinked (DHT) as a Cutaneous Substitute for Wound Healing
Abstract
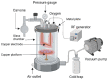
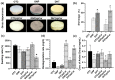
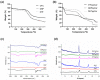
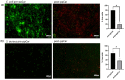
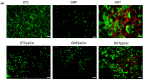
Tissue engineering products have grown in popularity as a therapeutic approach for chronic wounds and burns. However, some drawbacks include additional steps and a lack of antibacterial capacities, both of which need to be addressed to treat wounds effectively. This study aimed to develop an acellular, ready-to-use ovine tendon collagen type I (OTC-I) bioscaffold with an antibacterial coating for the immediate treatment of skin wounds and to prevent infection post-implantation. Two types of crosslinkers, 0.1% genipin (GNP) and dehydrothermal treatment (DHT), were explored to optimise the material strength and biodegradability compared with a non-crosslinked (OTC) control. Carvone plasma polymerisation (ppCar) was conducted to deposit an antibacterial protective coating. Various parameters were performed to investigate the physicochemical properties, mechanical properties, microstructures, biodegradability, thermal stability, surface wettability, antibacterial activity and biocompatibility of the scaffolds on human skin cells between the different crosslinkers, with and without plasma polymerisation. GNP is a better crosslinker than DHT because it demonstrated better physicochemical properties (27.33 ± 5.69% vs. 43 ± 7.64% shrinkage), mechanical properties (0.15 ± 0.15 MPa vs. 0.07 ± 0.08 MPa), swelling (2453 ± 419.2% vs. 1535 ± 392.9%), biodegradation (0.06 ± 0.06 mg/h vs. 0.15 ± 0.16 mg/h), microstructure and biocompatibility. Similarly, its ppCar counterpart, GNPppCar, presents promising results as a biomaterial with enhanced antibacterial properties. Plasma-polymerised carvone on a crosslinked collagen scaffold could also support human skin cell proliferation and viability while preventing infection. Thus, GNPppCar has potential for the rapid treatment of healing wounds.
Keywords: antibacterial; biomaterial; carvone; collagen; dehydrothermal treatment; genipin; plasma polymerisation; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Snyder D., Sullivan N., Margolis D., Schoelles K. Skin Substitutes for Treating Chronic Wounds [Internet] Agency for Healthcare Research and Quality; Rockville, MD, USA: 2020. - PubMed
Grants and funding
- UKM-TR-006/Universiti Kebangsaan Malaysia under Geran Translasi UKM (TR-UKM)
- IF0419Q1081./Ministry of Science, Technology and Innovation (MOSTI; Malaysia) provided reliable funds under International Collaboration Fund 910 (ICF)
- Agreement No:101008041/The European Union's Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant
LinkOut - more resources
Full Text Sources

