Photoelectrocatalytic Degradation of Methylene Blue on Electrodeposited Bismuth Ferrite Perovskite Films
- PMID: 37049063
- PMCID: PMC10095613
- DOI: 10.3390/ma16072769
Photoelectrocatalytic Degradation of Methylene Blue on Electrodeposited Bismuth Ferrite Perovskite Films
Abstract
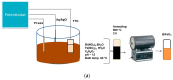
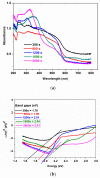
Electrodeposited bismuth ferrite (BiFeO3) thin films on fluorine-doped tin oxide (FTO) substrate were employed as photoanodes in the photoelectrocatalytic degradation of methylene blue. The BiFeO3 thin films electrodeposited for 300 s, 600 s, 1200 s, 1800 s and 3600 s were characterised with XRD, field emission scanning electron microscopy (FESEM) and UV-vis diffuse reflectance spectroscopy. SEM images displayed different morphology at different electrodeposition times which affects the photoelectrocatalytic (PEC) performances. The FESEM cross-sectional area was used to measure the thickness of the film. The optical properties showed that the band gaps of the photoanodes were increasing as the electrodeposition time increased. The photocurrent response obtained showed that all thin film photoanodes responded to visible light and lower charge transfer resistance (from electrochemical impedance spectroscopy studies) was observed with photoanodes electrodeposited at a shorter time compared to those at a longer time. The PEC application of the photoanode for the removal of methylene blue (MB) dye in water showed that the percentage degradation decreased with an increase in electrodeposition time with removal rates of 97.6% and 70% observed in 300 s and 3600 s electrodeposition time, respectively. The extent of mineralisation was measured by total organic carbon and reusability studies were carried out. Control experiments such as adsorption, photolysis, photocatalysis and electrocatalysis processes were also investigated in comparison with PEC. The electrodeposition approach with citric acid exhibited improved electrode stability while mitigating the problem of catalyst leaching or peeling off during the PEC process.
Keywords: bismuth ferrite perovskite; citric acid; electrodeposition; film thickness; methylene blue; photoelectrocatalytic degradation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











Similar articles
-
Enhanced photoelectrocatalytic performance of titanium dioxide/carbon cloth based photoelectrodes by graphene modification under visible-light irradiation.J Hazard Mater. 2013 Dec 15;263 Pt 2:291-8. doi: 10.1016/j.jhazmat.2013.09.013. Epub 2013 Sep 16. J Hazard Mater. 2013. PMID: 24091125
-
Enhanced Visible Light-Driven Photoelectrocatalytic Degradation of Paracetamol at a Ternary z-Scheme Heterojunction of Bi2WO6 with Carbon Nanoparticles and TiO2 Nanotube Arrays Electrode.Nanomaterials (Basel). 2022 Jul 19;12(14):2467. doi: 10.3390/nano12142467. Nanomaterials (Basel). 2022. PMID: 35889691 Free PMC article.
-
Carbon quantum dots-decorated TiO2/g-C3N4 film electrode as a photoanode with improved photoelectrocatalytic performance for 1,4-dioxane degradation.Chemosphere. 2020 Jul;251:126381. doi: 10.1016/j.chemosphere.2020.126381. Epub 2020 Feb 29. Chemosphere. 2020. PMID: 32443232
-
Design strategies and mechanisms of g-C3N4-based photoanodes for photoelectrocatalytic degradation of organic pollutants in water.J Environ Manage. 2023 Oct 15;344:118545. doi: 10.1016/j.jenvman.2023.118545. Epub 2023 Jul 5. J Environ Manage. 2023. PMID: 37418928 Review.
-
Nanostructured tungsten trioxide thin films synthesized for photoelectrocatalytic water oxidation: a review.ChemSusChem. 2014 Nov;7(11):2974-97. doi: 10.1002/cssc.201402089. Epub 2014 Oct 2. ChemSusChem. 2014. PMID: 25274424 Review.
Cited by
-
Zinc Oxide-Graphitic Carbon Nitride Composites: Synthesis, Properties, and Application Scopes in Environmental Remediations.Small. 2025 Sep;21(36):e09637. doi: 10.1002/smll.202409637. Epub 2025 Jul 25. Small. 2025. PMID: 40709811 Free PMC article. Review.
-
Interfacial Engineering of a Z-Scheme Bi2O2S/NiTiO3 Heterojunction Photoanode for the Degradation of Sulfamethoxazole in Water.ACS Appl Mater Interfaces. 2025 Jan 8;17(1):1385-1398. doi: 10.1021/acsami.4c20102. Epub 2024 Dec 5. ACS Appl Mater Interfaces. 2025. PMID: 39635741 Free PMC article.
References
-
- Din M.I., Khalid R., Najeeb J., Hussain Z. Fundamentals and photocatalysis of methylene blue dye using various nanocatalytic assemblies—A critical review. J. Clean. Prod. 2021;298:126567. doi: 10.1016/j.jclepro.2021.126567. - DOI
-
- Foghahazade N., Mousavi M., Behnejad H., Hamzehloo M., Habibi-Yangjeh A. Fabrication, characterization, and photocatalytic studies of novel ZnO/Ag3BiO3 nanocomposites: Impressive photocatalysts for degradation of some dyes. J. Mater. Sci. Mater. Electron. 2021;32:2704–2718. doi: 10.1007/s10854-020-04978-0. - DOI
-
- Kumar A., Kumar A., Krishnan V. Perovskite Oxide Based Materials for Energy and Environment-Oriented Photocatalysis. ACS Catal. 2020;10:10253–10315. doi: 10.1021/acscatal.0c02947. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

