Adsorbent Material Based on Carbon Black and Bismuth with Tunable Properties for Gold Recovery
- PMID: 37049135
- PMCID: PMC10096360
- DOI: 10.3390/ma16072837
Adsorbent Material Based on Carbon Black and Bismuth with Tunable Properties for Gold Recovery
Abstract
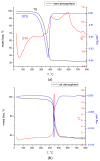
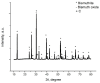
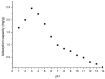
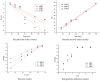
Adsorption recovery of precious metals on a variety of solid substrates has steadily gained increased attention in recent years. Special attention was paid to the studies on the characterization of the adsorptive properties of materials with a high affinity for gold depending on the nature of the pendant groups present in the structure of the material. The aim of the present work was to synthesize and characterize a new material by using the sol-gel synthesis method (designated as BCb/CB). In this case, synthesis involved the following precursors: bismuth carbonate (III), carbon black, and IGEPAL surfactant (octylphenoxypolyethoxyethanol). Immobilization of the heterojunction as bismuth oxide over a flexible support such as carbon black (CB) can prevent their elution in solution and make it versatile for its use in a system. In this work, a new adsorbent material based on bismuth carbonate supported over carbon black (BCb/CB) was developed and used further for gold recovery from aqueous solutions. The required material was characterized physically/chemically by scanning electron microscopy (SEM); energy dispersive X-ray spectrometry (EDX); X-ray diffraction (XRD); thermal analysis (DTG/DTA); atomic force microscopy (AFM). The Brunauer-Emmett-Teller (BET) method was used to determine the specific surface area indicating a value of approximately 40 m2/g, higher than the surface of CB precursor (36 m2/g). The adsorptive properties and the adsorption mechanism of the materials were highlighted in order to recover Au(III). For this, static adsorption studies were carried out. The parameters that influence the adsorption process were studied, namely: the pH, the contact time, the temperature, and the initial concentration of the gold ions in the used solution. In order to establish the mechanism of the adsorption process, kinetic, thermodynamic, and equilibrium studies were carried out. Experimental data proved that the gold recovery can be conducted with maximum performance at pH 3, at room temperature. Thermodynamic studies proved that the gold adsorption on BCb/CB material is a spontaneous and endothermal process. The results indicate a total adsorption capacity of 13.1 mg Au(III)/g material. By using this material in real solutions, a recovery efficiency of 90.5% was obtained, concomitant with a higher selectivity (around 95%).
Keywords: adsorption; bismuth (III) carbonate basic; carbon black; gold recovery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Reith F., Rea M.A.D., Sawley P., Zammit C.M., Nolze G., Reith T., Rantanen K., Bissett A. Biogeochemical cycling of gold: Transforming gold particles from arctic Finland. Chem. Geol. 2018;483:511–529. doi: 10.1016/j.chemgeo.2018.03.021. - DOI
-
- Philips G.N. Conglomerate-hosted gold deposits: What is so special? In Proceedings of the Gold18@Perth, An Australian Institute of Geoscientists symposium organised in conjunction with Geoscientists Symposia, Perth, WA, Australia, 2–3 August 2018;
-
- Saradesh K.M., Vinodkumar G.S. Metallurgical processes for hardening of 22Karat Gold for light weight and high strength jewelry manufacturing. J. Mater. Res. Technol. 2020;9:2009–2020. doi: 10.1016/j.jmrt.2019.12.033. - DOI
-
- Corti C.W. Metallurgy of Microalloyed 24 Carat Golds. Gold Bull. 1999;32:39–47. doi: 10.1007/BF03214789. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

