Nitrogen-Doped Graphene Oxide as Efficient Metal-Free Electrocatalyst in PEM Fuel Cells
- PMID: 37049326
- PMCID: PMC10096973
- DOI: 10.3390/nano13071233
Nitrogen-Doped Graphene Oxide as Efficient Metal-Free Electrocatalyst in PEM Fuel Cells
Abstract
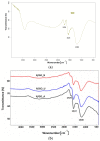
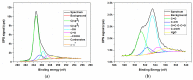
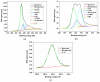
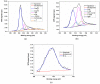
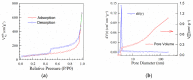
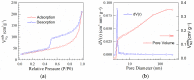
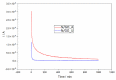
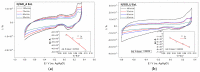
Nitrogen-doped graphene is currently recognized as one of the most promising catalysts for the oxygen reduction reaction (ORR). It has been demonstrated to act as a metal-free electrode with good electrocatalytic activity and long-term operation stability, excellent for the ORR in proton exchange membrane fuel cells (PEMFCs). As a consequence, intensive research has been dedicated to the investigation of this catalyst through varying the methodologies for the synthesis, characterization, and technologies improvement. A simple, scalable, single-step synthesis method for nitrogen-doped graphene oxide preparation was adopted in this paper. The physical and chemical properties of various materials obtained from different precursors have been evaluated and compared, leading to the conclusion that ammonia allows for a higher resulting nitrogen concentration, due to its high vapor pressure, which facilitates the functionalization reaction of graphene oxide. Electrochemical measurements indicated that the presence of nitrogen-doped oxide can effectively enhance the electrocatalytic activity and stability for ORR, making it a viable candidate for practical application as a PEMFC cathode electrode.
Keywords: electrocatalyst; long-term operation stability; metal-free; nitrogen-doped graphene oxide; oxygen reduction reaction; proton-exchange membrane fuel cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









Similar articles
-
Nitrogen-doped graphene as efficient metal-free electrocatalyst for oxygen reduction in fuel cells.ACS Nano. 2010 Mar 23;4(3):1321-6. doi: 10.1021/nn901850u. ACS Nano. 2010. PMID: 20155972
-
Covalent functionalization based heteroatom doped graphene nanosheet as a metal-free electrocatalyst for oxygen reduction reaction.Nanoscale. 2013 Dec 21;5(24):12255-60. doi: 10.1039/c3nr03581f. Nanoscale. 2013. PMID: 24146109
-
Fe-N4 Doped Carbon Nanotube Cathode Catalyst for PEM Fuel Cells.ACS Appl Mater Interfaces. 2021 Oct 20;13(41):48923-48933. doi: 10.1021/acsami.1c15554. Epub 2021 Oct 10. ACS Appl Mater Interfaces. 2021. PMID: 34628849
-
Non-Noble Metal Catalysts in Cathodic Oxygen Reduction Reaction of Proton Exchange Membrane Fuel Cells: Recent Advances.Nanomaterials (Basel). 2022 Sep 24;12(19):3331. doi: 10.3390/nano12193331. Nanomaterials (Basel). 2022. PMID: 36234459 Free PMC article. Review.
-
Pt-Based Oxygen Reduction Reaction Catalysts in Proton Exchange Membrane Fuel Cells: Controllable Preparation and Structural Design of Catalytic Layer.Nanomaterials (Basel). 2022 Nov 24;12(23):4173. doi: 10.3390/nano12234173. Nanomaterials (Basel). 2022. PMID: 36500796 Free PMC article. Review.
Cited by
-
Graphene nanocomposites for real-time electrochemical sensing of nitric oxide in biological systems.Appl Phys Rev. 2023 Nov 30;10:041310. doi: 10.1063/5.0162640. Appl Phys Rev. 2023. PMID: 38229764 Free PMC article.
-
Constructing In2S3/CdS/N-rGO Hybrid Nanosheets via One-Pot Pyrolysis for Boosting and Stabilizing Visible Light-Driven Hydrogen Evolution.Molecules. 2023 Nov 30;28(23):7878. doi: 10.3390/molecules28237878. Molecules. 2023. PMID: 38067607 Free PMC article.
References
-
- Chuanyu S., Huan Z. Review of the Development of First-Generation Redox Flow Batteries: Iron-Chromium System. ChemSusChem. 2022;15:202101798. - PubMed
-
- Ma R., Lin G., Zhou Y., Liu Q., Zhang T., Shan G. A review of oxygen reduction mechanisms for metal-free carbon-based electrocatalysts. Npj Comput. Mater. 2019;5:78. doi: 10.1038/s41524-019-0210-3. - DOI
-
- Sui S., Wang X., Zhou X., Su Y., Riffat S., Liu C. A comprehensive review of Pt electrocatalysts for the oxygen reduction reaction: Nanostructure, activity, mechanism, and carbon support in PEM fuel cells. J. Mater. Chem. A. 2017;5:1808–1825. doi: 10.1039/C6TA08580F. - DOI
-
- Kiani M., Tian X.Q., Zhang W. Non-precious metal electrocatalysts design for oxygen reduction reaction in polymer electrolyte membrane fuel cells: Recent advances, challenges and future perspectives. Coord. Chem. Rev. 2021;441:213954. doi: 10.1016/j.ccr.2021.213954. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

