One-Step Synthesis of a Binder-Free, Stable, and High-Performance Electrode; Cu-O|Cu3P Heterostructure for the Electrocatalytic Methanol Oxidation Reaction (MOR)
- PMID: 37049328
- PMCID: PMC10096724
- DOI: 10.3390/nano13071234
One-Step Synthesis of a Binder-Free, Stable, and High-Performance Electrode; Cu-O|Cu3P Heterostructure for the Electrocatalytic Methanol Oxidation Reaction (MOR)
Abstract
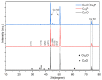
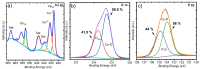
Although direct methanol fuel cells (DMFCs) have been spotlighted in the past decade, their commercialization has been hampered by the poor efficiency of the methanol oxidation reaction (MOR) due to the unsatisfactory performance of currently available electrocatalysts. Herein, we developed a binder-free, copper-based, self-supported electrode consisting of a heterostructure of Cu3P and mixed copper oxides, i.e., cuprous-cupric oxide (Cu-O), as a high-performance catalyst for the electro-oxidation of methanol. We synthesized a self-supported electrode composed of Cu-O|Cu3P using a two-furnace atmospheric pressure-chemical vapor deposition (AP-CVD) process. High-resolution transmission electron microscopy analysis revealed the formation of 3D nanocrystals with defects and pores. Cu-O|Cu3P outperformed the MOR activity of individual Cu3P and Cu-O owing to the synergistic interaction between them. Cu3P|Cu-O exhibited a highest anodic current density of 232.5 mAcm-2 at the low potential of 0.65 V vs. Hg/HgO, which is impressive and superior to the electrocatalytic activity of its individual counterparts. The formation of defects, 3D morphology, and the synergistic effect between Cu3P and Cu-O play a crucial role in facilitating the electron transport between electrode and electrolyte to obtain the optimal MOR activity. Cu-O|Cu3P shows outstanding MOR stability for about 3600 s with 100% retention of the current density, which proves its robustness alongside CO intermediate.
Keywords: chemical vapor deposition; heterostructure; methanol oxidation reaction; self-standing electrode; synergistic effect.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Phosphorization Engineering on a MOF-Derived Metal Phosphide Heterostructure (Cu/Cu3P@NC) as an Electrode for Enhanced Supercapacitor Performance.Inorg Chem. 2023 Oct 23;62(42):17083-17092. doi: 10.1021/acs.inorgchem.3c01440. Epub 2023 Oct 11. Inorg Chem. 2023. PMID: 37820058
-
Highly selective electrochemical nitrate reduction using copper phosphide self-supported copper foam electrode: Performance, mechanism, and application.Water Res. 2021 Apr 1;193:116881. doi: 10.1016/j.watres.2021.116881. Epub 2021 Jan 28. Water Res. 2021. PMID: 33571901
-
Cu3P/CoP Heterostructure for Efficient Electrosynthesis of Ammonia from Nitrate Reduction Reaction.ACS Appl Mater Interfaces. 2025 Jan 8;17(1):980-990. doi: 10.1021/acsami.4c16144. Epub 2024 Dec 18. ACS Appl Mater Interfaces. 2025. PMID: 39693253
-
Toward Electrocatalytic Methanol Oxidation Reaction: Longstanding Debates and Emerging Catalysts.Adv Mater. 2023 Jun;35(26):e2211099. doi: 10.1002/adma.202211099. Epub 2023 May 28. Adv Mater. 2023. PMID: 36706444 Review.
-
Recent advancements and prospects in noble and non-noble electrocatalysts for materials methanol oxidation reactions.Discov Nano. 2024 Aug 14;19(1):128. doi: 10.1186/s11671-024-04066-w. Discov Nano. 2024. PMID: 39143373 Free PMC article. Review.
References
-
- Malik B., Vijaya Sankar K., Aziz S.K.T., Majumder S., Tsur Y., Nessim G.D. Uncovering the Change in Catalytic Activity during Electro-oxidation of Urea: Answering Overisolation of the Relaxation Phenomenon. J. Phys. Chem. C. 2021;125:23126–23132. doi: 10.1021/acs.jpcc.1c06059. - DOI
-
- Kundu S., Malik B., Pattanayak D.K., Pillai V.K. Mixed Valent, Distorted Cobalt Ludwigite (Co3BO5/Co3O2BO3) and Its Composite with Reduced Multiwalled Carbon Nanotubes (R-MWCNT) in Enhancing the Domain Edge-Sharing Oxygen as Superior Water Oxidation Electrocatalysts. ChemElectroChem. 2018;5:1670–1676. doi: 10.1002/celc.201800389. - DOI
-
- Kundu S., Malik B., Pattanayak D.K., Ragupathy P., Pillai V.K. Role of Specific N-Containing Active Sites in Interconnected Graphene Quantum Dots for the Enhanced Electrocatalytic Activity towards Oxygen Evolution Reaction. ChemistrySelect. 2017;2:9943–9946. doi: 10.1002/slct.201701952. - DOI
-
- Khalid M., De B.S., Singh A., Shahgaldi S. Lignin Electrolysis at Room Temperature on Nickel Foam for Hydrogen Generation: Performance Evaluation and Effect of Flow Rate. Catalysts. 2022;12:1646. doi: 10.3390/catal12121646. - DOI
-
- Luo J., Hu F., Xi B., Han Q., Wu X., Wu Y. Fabricating of Ni-BTC/NiS 2 heterostructure via self-assembly strategy for electrocatalytic methanol oxidation. Inorg. Chem. Commun. 2022;143:109777. doi: 10.1016/j.inoche.2022.109777. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

