A Novel Drastic Peptide Genetically Adapted to Biomimetic Scaffolds "Delivers" Osteogenic Signals to Human Mesenchymal Stem Cells
- PMID: 37049329
- PMCID: PMC10096854
- DOI: 10.3390/nano13071236
A Novel Drastic Peptide Genetically Adapted to Biomimetic Scaffolds "Delivers" Osteogenic Signals to Human Mesenchymal Stem Cells
Abstract
This work describes the design, preparation, and deep investigation of "intelligent nanobiomaterials" that fulfill the safety rules and aim to serve as "signal deliverers" for osteogenesis, harboring a specific peptide that promotes and enhances osteogenesis at the end of their hydrogel fibers. The de novo synthesized protein fibers, besides their mechanical properties owed to their protein constituents from elastin, silk fibroin and mussel-foot adhesive protein-1 as well as to cell-attachment peptides from extracellular matrix glycoproteins, incorporate the Bone Morphogenetic Protein-2 (BMP2) peptide (AISMLYLDEN) that, according to our studies, serves as "signal deliverer" for osteogenesis. The osteogenetic capacity of the biomaterial has been evidenced by investigating the osteogenic marker genes ALP, RUNX2, Osteocalcin, COL1A1, BMPR1A, and BMPR2, which were increased drastically in cells cultured on scaffold-BMP2 for 21 days, even in the absence of osteogenesis medium. In addition, the induction of phosphorylation of intracellular Smad-1/5 and Erk-1/2 proteins clearly supported the osteogenetic capacity of the biomaterial.
Keywords: BMP-2 peptide; biomaterial; bone regeneration; elastin; mussel-foot protein; osteogenesis; scaffold; silk fibroin; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









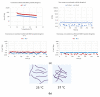

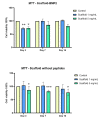

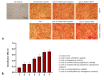

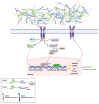
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

