A Review of Analytical Techniques for the Determination and Separation of Silver Ions and Its Nanoparticles
- PMID: 37049355
- PMCID: PMC10097010
- DOI: 10.3390/nano13071262
A Review of Analytical Techniques for the Determination and Separation of Silver Ions and Its Nanoparticles
Abstract
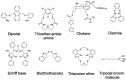
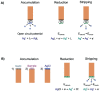
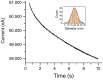
Many articles have already been published dealing with silver ions and its nanoparticles, but mostly from the environmental and toxicological point of view. This article is a review focused on the various analytical techniques and detection platforms used in the separation and determination of mentioned above species, especially on the trace concentration level. Commonly used are optical methods because of their high sensitivity and easy automation. The separation methods are mainly used for the separation and preconcentration of silver particles. Their combination with other analytical techniques, mainly inductively coupled plasma mass spectrometry (ICP-MS) leads to very low detection limits of analysis. The electrochemical methods are also powerful and perspective mainly because of the fabrication of new sensors designed for silver determination. All methods may be combined with each other to achieve a synergistic improvement of analytical parameters with an impact on sensitivity, selectivity and reliability. The paper comprises a review of all three types of analytical methods on the determination of trace quantities of silver ions and its nanoparticles.
Keywords: AgNPs; analytical methods; silver; silver detection; silver nanoparticles; trace amounts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Xu K., Pérez-Ráfols C., Cuartero M., Crespo G.A. Electrochemical Detection of Trace Silver. Electrochim. Acta. 2021;374:137929. doi: 10.1016/j.electacta.2021.137929. - DOI
-
- Umapathi R., Raju C.V., Ghoreishian S.M., Rani G.M., Kumar K., Oh M.H., Park J.P., Huh Y.S. Recent advances in th use of graphitic carbon nitride-based composites for the electrochemical detection of hazardous contaminants. Coord. Chem. Rev. 2022;470:214708. doi: 10.1016/j.ccr.2022.214708. - DOI
-
- Barriada J.L., Tappin A.D., Evans E.H., Achterberg E.P. Dissolved Silver Measurements in Seawater. TrAC Trends Anal. Chem. 2007;26:809–817. doi: 10.1016/j.trac.2007.06.004. - DOI
Publication types
LinkOut - more resources
Full Text Sources

