Combined Effects of ESRα DNA Methylation and Progesterone on Glucose Metabolic Disorders: The Henan Rural Cohort Study
- PMID: 37049500
- PMCID: PMC10096615
- DOI: 10.3390/nu15071659
Combined Effects of ESRα DNA Methylation and Progesterone on Glucose Metabolic Disorders: The Henan Rural Cohort Study
Abstract
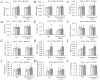
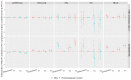
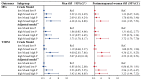
To explore the independent and combined effects of ESRα methylation and progesterone on impaired fasting glucose (IFG) and type 2 diabetes mellitus (T2DM), a case-control study including 901 subjects was conducted. Generalized linear models were performed to assess the independent and combined effects of ESRα methylation and progesterone on IFG or T2DM. Methylation level of cytosine-phosphoguanine (CpG) 1 in the estrogen receptor α (ESRα) gene was positively related to IFG in both men (odds ratio (OR) (95% confidence interval (CI)): 1.77 (1.05, 3.00)) and postmenopausal women (OR (95% CI): 1.82 (1.09, 3.04)), whereas the association between CpG 1 and T2DM was not significant. Positive associations of progesterone with IFG and T2DM were observed in both men (OR (95% CI): 2.03 (1.18, 3.49) and 3.00 (1.63, 5.52)) and postmenopausal women (OR (95% CI): 2.13 (1.27, 3.56) and 3.30 (1.85, 5.90)). Participants with high CpG 1 methylation plus high progesterone had an increased risk of IFG and T2DM, both in men and postmenopausal women. ESRα methylation and progesterone were positively associated with IFG, and the positive association between progesterone and T2DM was also found. Importantly, we firstly found the combined effects of ESRα methylation and progesterone on IFG and T2DM.
Keywords: DNA methylation progesterone; combined effect; estrogen receptor alpha; impaired fasting glucose; type 2 diabetes mellitus.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
References
-
- Magliano D.J., Boyko E.J. IDF Diabetes Atlas 10th Edition Scientific Committee. International Diabetes Federation; Brussels, Belgium: 2021. IDF Diabetes Atlas. - PubMed
-
- Heianza Y., Hara S., Arase Y., Saito K., Fujiwara K., Tsuji H., Kodama S., Hsieh S.D., Mori Y., Shimano H., et al. HbA1c 5·7–6·4% and impaired fasting plasma glucose for diagnosis of prediabetes and risk of progression to diabetes in Japan (TOPICS 3): A longitudinal cohort study. Lancet. 2011;378:147–155. doi: 10.1016/S0140-6736(11)60472-8. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- 42177415/National Natural Science Foundation of China
- 21806146/National Natural Science Foundation of China
- 22HASTIT044/Scientific and Technological Innovation of Colleges and Universities in Henan Province Talent Support Program
- 2020T130604/China Postdoctoral Science Foundation
- 2021M702934/China Postdoctoral Science Foundation
LinkOut - more resources
Full Text Sources
Medical

