The Protective Effect of 11-Keto-β-Boswellic Acid against Diabetic Cardiomyopathy in Rats Entails Activation of AMPK
- PMID: 37049501
- PMCID: PMC10097356
- DOI: 10.3390/nu15071660
The Protective Effect of 11-Keto-β-Boswellic Acid against Diabetic Cardiomyopathy in Rats Entails Activation of AMPK
Abstract
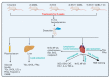
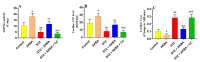
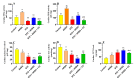
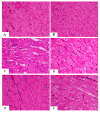
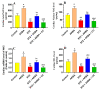
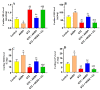
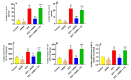
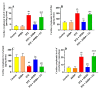
This study examined the protective effect of 11-keto-β-boswellic acid (AKBA) against streptozotocin (STZ)-induced diabetic cardiomyopathy (DC) in rats and examined the possible mechanisms of action. Male rats were divided into 5 groups (n = 8/each): (1) control, AKBA (10 mg/kg, orally), STZ (65 mg/kg, i.p.), STZ + AKBA (10 mg/kg, orally), and STZ + AKBA + compound C (CC/an AMPK inhibitor, 0.2 mg/kg, i.p.). AKBA improved the structure and the systolic and diastolic functions of the left ventricles (LVs) of STZ rats. It also attenuated the increase in plasma glucose, plasma insulin, and serum and hepatic levels of triglycerides (TGs), cholesterol (CHOL), and free fatty acids (FFAs) in these diabetic rats. AKBA stimulated the ventricular activities of phosphofructokinase (PFK), pyruvate dehydrogenase (PDH), and acetyl CoA carboxylase (ACC); increased levels of malonyl CoA; and reduced levels of carnitine palmitoyltransferase I (CPT1), indicating improvement in glucose and FA oxidation. It also reduced levels of malondialdehyde (MDA); increased mitochondria efficiency and ATP production; stimulated mRNA, total, and nuclear levels of Nrf2; increased levels of glutathione (GSH), heme oxygenase (HO-1), superoxide dismutase (SOD), and catalase (CAT); but reduced the expression and nuclear translocation of NF-κB and levels of tumor-necrosis factor-α (TNF-α) and interleukin-6 (IL-6). These effects were concomitant with increased activities of AMPK in the LVs of the control and STZ-diabetic rats. Treatment with CC abolished all these protective effects of AKBA. In conclusion, AKBA protects against DC in rats, mainly by activating the AMPK-dependent control of insulin release, cardiac metabolism, and antioxidant and anti-inflammatory effects.
Keywords: 11-keto-β-boswellic acid; AMPK; Nrf2; diabetic cardiomyopathy; oxidative stress; streptozotocin.
Conflict of interest statement
The are no conflicts of interest associated with this work.
Figures
Similar articles
-
Esculeoside A Decreases Diabetic Cardiomyopathy in Streptozotocin-Treated Rats by Attenuating Oxidative Stress, Inflammation, Fibrosis, and Apoptosis: Impressive Role of Nrf2.Medicina (Kaunas). 2023 Oct 14;59(10):1830. doi: 10.3390/medicina59101830. Medicina (Kaunas). 2023. PMID: 37893548 Free PMC article.
-
Isosteviol attenuates streptozotocin-mediated diabetic nephropathy in rats by upregulating and stimulating adenosine monophosphate-activated protein kinase.J Physiol Pharmacol. 2023 Jun;74(3). doi: 10.26402/jpp.2023.3.06. Epub 2023 Aug 30. J Physiol Pharmacol. 2023. PMID: 37661183
-
Kaempferol protects against streptozotocin-induced diabetic cardiomyopathy in rats by a hypoglycemic effect and upregulating SIRT1.J Physiol Pharmacol. 2021 Jun;72(3). doi: 10.26402/jpp.2021.3.04. Epub 2021 Nov 19. J Physiol Pharmacol. 2021. PMID: 34810287
-
Phloretamide Prevent Hepatic and Pancreatic Damage in Diabetic Male Rats by Modulating Nrf2 and NF-κB.Nutrients. 2023 Mar 17;15(6):1456. doi: 10.3390/nu15061456. Nutrients. 2023. PMID: 36986192 Free PMC article.
-
Boswellic extracts and 11-keto-ß-boswellic acids prevent type 1 and type 2 diabetes mellitus by suppressing the expression of proinflammatory cytokines.Phytomedicine. 2019 Oct;63:153002. doi: 10.1016/j.phymed.2019.153002. Epub 2019 Jun 28. Phytomedicine. 2019. PMID: 31301539 Review.
Cited by
-
Antidiabetic and antioxidant properties of Boswellia sacra oleo-gum in streptozotocin-induced diabetic rats.J Ayurveda Integr Med. 2024 Jul-Aug;15(4):101014. doi: 10.1016/j.jaim.2024.101014. Epub 2024 Aug 20. J Ayurveda Integr Med. 2024. PMID: 39167989 Free PMC article.
-
A comprehensive review on diabetic cardiomyopathy (DCM): histological spectrum, diagnosis, pathogenesis, and management with conventional treatments and natural compounds.Naunyn Schmiedebergs Arch Pharmacol. 2025 Aug;398(8):9929-9969. doi: 10.1007/s00210-025-03980-9. Epub 2025 Mar 18. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40100371 Free PMC article. Review.
-
Periplaneta americana extract improves recurrent oral ulcers through regulation of TLR4/NF-κB and Nrf2/HO-1 pathways.Sci Rep. 2025 Mar 12;15(1):8578. doi: 10.1038/s41598-024-84703-7. Sci Rep. 2025. PMID: 40075107 Free PMC article.
-
Esculeoside A Decreases Diabetic Cardiomyopathy in Streptozotocin-Treated Rats by Attenuating Oxidative Stress, Inflammation, Fibrosis, and Apoptosis: Impressive Role of Nrf2.Medicina (Kaunas). 2023 Oct 14;59(10):1830. doi: 10.3390/medicina59101830. Medicina (Kaunas). 2023. PMID: 37893548 Free PMC article.
-
Acetyl-11-keto-β-boswellic acid restrains the progression of synovitis in osteoarthritis via the Nrf2/HO-1 pathway.Acta Biochim Biophys Sin (Shanghai). 2024 Jul 8;56(11):1644-1658. doi: 10.3724/abbs.2024102. Acta Biochim Biophys Sin (Shanghai). 2024. PMID: 38982914 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

