In Vitro Anti-Inflammatory and Vasculoprotective Effects of Red Cell Extract from the Black Sea Urchin Arbacia lixula
- PMID: 37049512
- PMCID: PMC10096920
- DOI: 10.3390/nu15071672
In Vitro Anti-Inflammatory and Vasculoprotective Effects of Red Cell Extract from the Black Sea Urchin Arbacia lixula
Abstract
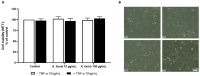
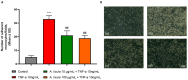
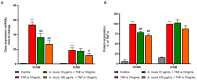
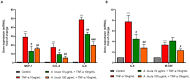
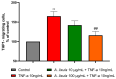
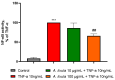
Sea urchins have emerged as an important source of bioactive compounds with anti-inflammatory and antioxidant properties relevant to human health. Since inflammation is a crucial pathogenic process in the development and progression of atherosclerosis, we here assessed the potential anti-inflammatory and vasculoprotective effects of coelomic red-cell methanolic extract of the black sea urchin Arbacia lixula in an in vitro model of endothelial cell dysfunction. Human microvascular endothelial cells (HMEC-1) were pretreated with A. lixula red-cell extract (10 and 100 μg/mL) before exposure to the pro-inflammatory cytokine tumor necrosis factor (TNF)-α. The extract was non-toxic after 24 h cell treatment and was characterized by antioxidant power and phenol content. The TNF-α-stimulated expression of adhesion molecules (VCAM-1, ICAM-1) and cytokines/chemokines (MCP-1, CCL-5, IL-6, IL-8, M-CSF) was significantly attenuated by A. lixula red-cell extract. This was functionally accompanied by a reduction in monocyte adhesion and chemotaxis towards activated endothelial cells. At the molecular level, the tested extract significantly counteracted the TNF-α-stimulated activation of the pro-inflammatory transcription factor NF-κB. These results provide evidence of potential anti-atherosclerotic properties of A. lixula red-cell extract, and open avenues in the discovery and development of dietary supplements and/or drugs for the prevention or treatment of cardiovascular diseases.
Keywords: NF-κB; adhesion molecule; atherosclerosis; chemokine; cytokine; endothelial dysfunction; gene expression; inflammation; monocyte adhesion; red cells; sea urchin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Anti-atherogenic mechanism of ethanol extract of Christia vespertilionis (L.f.) Bakh. F. Leaves in vitro.Int Immunopharmacol. 2024 Jun 15;134:112148. doi: 10.1016/j.intimp.2024.112148. Epub 2024 May 7. Int Immunopharmacol. 2024. PMID: 38718657
-
Gynura procumbens (Lour.) Merr. extract attenuates monocyte adherence to endothelial cells through suppression of the NF-κB signaling pathway.J Ethnopharmacol. 2022 Aug 10;294:115391. doi: 10.1016/j.jep.2022.115391. Epub 2022 May 16. J Ethnopharmacol. 2022. PMID: 35589022
-
Chloroform extract of aged black garlic attenuates TNF-α-induced ROS generation, VCAM-1 expression, NF-κB activation and adhesiveness for monocytes in human umbilical vein endothelial cells.Phytother Res. 2011 Jan;25(1):92-100. doi: 10.1002/ptr.3230. Phytother Res. 2011. PMID: 20623600
-
Multiple anti-inflammatory and anti-atherosclerotic properties of red wine polyphenolic extracts: differential role of hydroxycinnamic acids, flavonols and stilbenes on endothelial inflammatory gene expression.Eur J Nutr. 2016 Mar;55(2):477-489. doi: 10.1007/s00394-015-0865-6. Epub 2015 Feb 28. Eur J Nutr. 2016. PMID: 25724173
-
Antioxidants and Atherosclerosis: Mechanistic Aspects.Biomolecules. 2019 Jul 25;9(8):301. doi: 10.3390/biom9080301. Biomolecules. 2019. PMID: 31349600 Free PMC article. Review.
Cited by
-
In silico analysis of Arbacia lixula-derived peptides and plasmid construction for recombinant anti-aging therapies.Narra J. 2024 Dec;4(3):e1283. doi: 10.52225/narra.v4i3.1283. Epub 2024 Nov 20. Narra J. 2024. PMID: 39816070 Free PMC article.
References
-
- Mayer A.M., Rodriguez A.D., Taglialatela-Scafati O., Fusetani N. Marine pharmacology in 2009–2011: Marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Mar. Drugs. 2013;11:2510–2573. doi: 10.3390/md11072510. - DOI - PMC - PubMed
-
- Stabili L., Acquaviva M.I., Cavallo R.A., Gerardi C., Narracci M., Pagliara P. Screening of Three Echinoderm Species as New Opportunity for Drug Discovery: Their Bioactivities and Antimicrobial Properties. Evid. Based Complement. Altern. Med. 2018;2018:7891748. doi: 10.1155/2018/7891748. - DOI - PMC - PubMed
-
- Moreno-Garcia D.M., Salas-Rojas M., Fernandez-Martinez E., Lopez-Cuellar M.D.R., Sosa-Gutierrez C.G., Pelaez-Acero A., Rivero-Perez N., Zaragoza-Bastida A., Ojeda-Ramirez D. Sea urchins: An update on their pharmacological properties. PeerJ. 2022;10:E13606. doi: 10.7717/peerj.13606. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

