Renal-Protective Roles of Lipoic Acid in Kidney Disease
- PMID: 37049574
- PMCID: PMC10097220
- DOI: 10.3390/nu15071732
Renal-Protective Roles of Lipoic Acid in Kidney Disease
Abstract
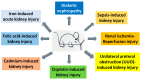
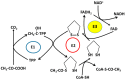
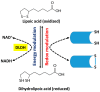
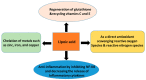
The kidney is a crucial organ that eliminates metabolic waste and reabsorbs nutritious elements. It also participates in the regulation of blood pressure, maintenance of electrolyte balance and blood pH homeostasis, as well as erythropoiesis and vitamin D maturation. Due to such a heavy workload, the kidney is an energy-demanding organ and is constantly exposed to endogenous and exogenous insults, leading to the development of either acute kidney injury (AKI) or chronic kidney disease (CKD). Nevertheless, there are no therapeutic managements to treat AKI or CKD effectively. Therefore, novel therapeutic approaches for fighting kidney injury are urgently needed. This review article discusses the role of α-lipoic acid (ALA) in preventing and treating kidney diseases. We focus on various animal models of kidney injury by which the underlying renoprotective mechanisms of ALA have been unraveled. The animal models covered include diabetic nephropathy, sepsis-induced kidney injury, renal ischemic injury, unilateral ureteral obstruction, and kidney injuries induced by folic acid and metals such as cisplatin, cadmium, and iron. We highlight the common mechanisms of ALA's renal protective actions that include decreasing oxidative damage, increasing antioxidant capacities, counteracting inflammation, mitigating renal fibrosis, and attenuating nephron cell death. It is by these mechanisms that ALA achieves its biological function of alleviating kidney injury and improving kidney function. Nevertheless, we also point out that more comprehensive, preclinical, and clinical studies will be needed to make ALA a better therapeutic agent for targeting kidney disorders.
Keywords: acute kidney injury; chronic kidney disease; diabetic kidney disease; diabetic nephropathy; lipoic acid; nephroprotection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
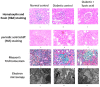

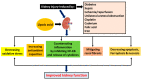
References
-
- Rennke H.G., Denker B.M. Renal Pathology: The Essentials. 5th ed. Wolters Kluwer; New York, NY, USA: 2020.
-
- Koeppen B.M., Stanton B.A. Renal Physiology. 5th ed. Elsevier; Philadelphia, PA, USA: 2013.
-
- Dipiro J.T., Talbet R.L., Yee G.C., Matzke G.R., Wells B.G., Posey L.M. Pharmacotherapy: A Pathophysiological Approach. 9th ed. McGraw-Hill Education; New York, NY, USA: 2014.
-
- Lieberman M., Marks A.D. Marks’ Basic Medical Biochemistry: A Clinical Approach. 4th ed. Wolters Kluwer/Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2013.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

