Is Autologous Fecal Microbiota Transfer after Exclusive Enteral Nutrition in Pediatric Crohn's Disease Patients Rational and Feasible? Data from a Feasibility Test
- PMID: 37049583
- PMCID: PMC10096730
- DOI: 10.3390/nu15071742
Is Autologous Fecal Microbiota Transfer after Exclusive Enteral Nutrition in Pediatric Crohn's Disease Patients Rational and Feasible? Data from a Feasibility Test
Abstract
Background: Exclusive enteral nutrition (EEN) is a highly effective therapy for remission induction in pediatric Crohn's disease (CD), but relapse rates after return to a regular diet are high. Autologous fecal microbiota transfer (FMT) using stool collected during EEN-induced clinical remission might represent a novel approach to maintaining the benefits of EEN.
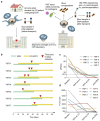
Methods: Pediatric CD patients provided fecal material at home, which was shipped at 4 °C to an FMT laboratory for FMT capsule generation and extensive pathogen safety screening. The microbial community composition of samples taken before and after shipment and after encapsulation was characterized using 16S rRNA amplicon sequencing.
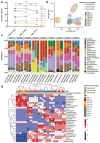
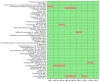
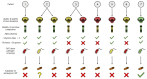
Results: Seven pediatric patients provided fecal material for nine test runs after at least three weeks of nutritional therapy. FMT capsules were successfully generated in 6/8 deliveries, but stool weight and consistency varied widely. Transport and processing of fecal material into FMT capsules did not fundamentally change microbial composition, but microbial richness was <30 genera in 3/9 samples. Stool safety screening was positive for potential pathogens or drug resistance genes in 8/9 test runs.
Conclusions: A high pathogen burden, low-diversity microbiota, and practical deficiencies of EEN-conditioned fecal material might render autologous capsule-FMT an unsuitable approach as maintenance therapy for pediatric CD patients.
Keywords: Crohn’s disease; autologous FMT; exclusive enteral nutrition; fecal microbiota transfer; pediatric IBD.
Conflict of interest statement
T.S. received speaker fees from MSD and Nutricia (Danone). K.N. collaborates with Hipp (Pfaffenhofen, Germany) about novel probiotic strains. The rest of the authors declare that they have no potential conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- Kelly C.R., Yen E.F., Grinspan A.M., Kahn S.A., Atreja A., Lewis J.D., Moore T.A., Rubin D.T., Kim A.M., Serra S., et al. Fecal Microbiota Transplantation Is Highly Effective in Real-World Practice: Initial Results From the FMT National Registry. Gastroenterology. 2021;160:183–192.e3. doi: 10.1053/j.gastro.2020.09.038. - DOI - PMC - PubMed
-
- Aggarwala V., Mogno I., Li Z., Yang C., Britton G.J., Chen-Liaw A., Mitcham J., Bongers G., Gevers D., Clemente J.C., et al. Precise Quantification of Bacterial Strains after Fecal Microbiota Transplantation Delineates Long-Term Engraftment and Explains Outcomes. Nat. Microbiol. 2021;6:1309–1318. doi: 10.1038/s41564-021-00966-0. - DOI - PMC - PubMed
-
- Staley C., Kaiser T., Vaughn B.P., Graiziger C., Hamilton M.J., Kabage A.J., Khoruts A., Sadowsky M.J. Durable Long-Term Bacterial Engraftment Following Encapsulated Fecal Microbiota Transplantation To Treat Clostridium Difficile Infection. mBio. 2019;10:e01586-19. doi: 10.1128/mBio.01586-19. - DOI - PMC - PubMed
-
- Moayyedi P., Surette M.G., Kim P.T., Libertucci J., Wolfe M., Onischi C., Armstrong D., Marshall J.K., Kassam Z., Reinisch W., et al. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology. 2015;149:102–109.e6. doi: 10.1053/j.gastro.2015.04.001. - DOI - PubMed
-
- Paramsothy S., Kamm M.A., Kaakoush N.O., Walsh A.J., van den Bogaerde J., Samuel D., Leong R.W.L., Connor S., Ng W., Paramsothy R., et al. Multidonor Intensive Faecal Microbiota Transplantation for Active Ulcerative Colitis: A Randomised Placebo-Controlled Trial. Lancet. 2017;389:1218–1228. doi: 10.1016/S0140-6736(17)30182-4. - DOI - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

