Glyoxylic Acid, an α-Keto Acid Metabolite Derived from Glycine, Promotes Myogenesis in C2C12 Cells
- PMID: 37049603
- PMCID: PMC10096605
- DOI: 10.3390/nu15071763
Glyoxylic Acid, an α-Keto Acid Metabolite Derived from Glycine, Promotes Myogenesis in C2C12 Cells
Abstract
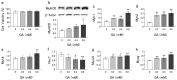
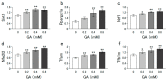
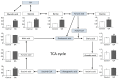
α-Keto acids may help prevent malnutrition in patients with chronic kidney disease (CKD), who consume protein-restricted diets, because they serve as amino acid sources without producing nitrogenous waste compounds. However, the physiological roles of α-keto acids, especially those derived from non-essential amino acids, remain unclear. In this study, we examined the effect of glyoxylic acid (GA), an α-keto acid metabolite derived from glycine, on myogenesis in C2C12 cells. Differentiation and mitochondrial biogenesis were used as myogenesis indicators. Treatment with GA for 6 d resulted in an increase in the expression of differentiation markers (myosin heavy chain II and myogenic regulatory factors), mitochondrial biogenesis, and intracellular amounts of amino acids (glycine, serine, and alanine) and their metabolites (citric acid and succinic acid). In addition, GA treatment suppressed the 2.5-µM dexamethasone (Dex)-induced increase in mRNA levels of ubiquitin ligases (Trim63 and Fbxo32), muscle atrophy markers. These results indicate that GA promotes myogenesis, suppresses Dex-induced muscle atrophy, and is metabolized to amino acids in muscle cells. Although further in vivo experiments are needed, GA may be a beneficial nutrient for ameliorating the loss of muscle mass, strength, and function in patients with CKD on a strict dietary protein restriction.
Keywords: C2C12 cells; glyoxylic acid; muscle atrophy; myogenesis; α-keto acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
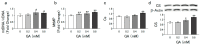

Similar articles
-
Keto Acids Attenuate Skeletal Muscle Atrophy in Chronic Kidney Disease via Inhibiting Pyroptosis and Upregulating Irisin Precursor FNDC5 Expression.Calcif Tissue Int. 2025 Apr 24;116(1):63. doi: 10.1007/s00223-025-01372-y. Calcif Tissue Int. 2025. PMID: 40272551
-
Dietary supplementation of branched-chain amino acids increases muscle net amino acid fluxes through elevating their substrate availability and intramuscular catabolism in young pigs.Br J Nutr. 2017 Apr;117(7):911-922. doi: 10.1017/S0007114517000757. Epub 2017 Apr 27. Br J Nutr. 2017. PMID: 28446262 Clinical Trial.
-
Lnc-ORA interacts with microRNA-532-3p and IGF2BP2 to inhibit skeletal muscle myogenesis.J Biol Chem. 2021 Jan-Jun;296:100376. doi: 10.1016/j.jbc.2021.100376. Epub 2021 Feb 4. J Biol Chem. 2021. PMID: 33548229 Free PMC article.
-
Mitochondrial Apoptotic Signaling Involvement in Remodeling During Myogenesis and Skeletal Muscle Atrophy.Semin Cell Dev Biol. 2023 Jul 15;143:66-74. doi: 10.1016/j.semcdb.2022.01.011. Epub 2022 Feb 28. Semin Cell Dev Biol. 2023. PMID: 35241367 Review.
-
[A Cellular Pharmacological Approach to the Development of Drugs to Treat Muscle Wasting].Yakugaku Zasshi. 2018;138(10):1271-1275. doi: 10.1248/yakushi.18-00091-3. Yakugaku Zasshi. 2018. PMID: 30270271 Review. Japanese.
Cited by
-
Causal Relationship Between Circulating Metabolites and Sarcopenia-Related Traits: A Mendelian Randomization and Experimental Study.Food Sci Nutr. 2025 Jan 9;13(1):e4624. doi: 10.1002/fsn3.4624. eCollection 2025 Jan. Food Sci Nutr. 2025. PMID: 39803274 Free PMC article.
-
Blackcurrant extract promotes differentiation of MC3T3‑E1 pre‑osteoblasts.Biomed Rep. 2024 Jun 19;21(2):121. doi: 10.3892/br.2024.1810. eCollection 2024 Aug. Biomed Rep. 2024. PMID: 38978537 Free PMC article.
-
Impact of glyphosate (RoundupTM) on the composition and functionality of the gut microbiome.Gut Microbes. 2023 Dec;15(2):2263935. doi: 10.1080/19490976.2023.2263935. Epub 2023 Oct 6. Gut Microbes. 2023. PMID: 38099711 Free PMC article. Review.
-
Palm kernel meal regulates the expression of genes involved in the amino acid metabolism in the liver of Tibetan sheep.BMC Vet Res. 2024 Jul 23;20(1):333. doi: 10.1186/s12917-024-04193-7. BMC Vet Res. 2024. PMID: 39044234 Free PMC article.
References
-
- Milan A.M., D’Souza R.F., Pundir S., Pileggi C.A., Thorstensen E.B., Barnett M.P., Markworth J.F., Cameron-Smith D., Mitchell C.J. Older Adults Have Delayed Amino Acid Absorption after a High Protein Mixed Breakfast Meal. J. Nutr. Health Aging. 2015;19:839–845. doi: 10.1007/s12603-015-0500-5. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

