Potential Effect of Glutamine in the Improvement of Intestinal Stem Cell Proliferation and the Alleviation of Burn-Induced Intestinal Injury via Activating YAP: A Preliminary Study
- PMID: 37049605
- PMCID: PMC10097377
- DOI: 10.3390/nu15071766
Potential Effect of Glutamine in the Improvement of Intestinal Stem Cell Proliferation and the Alleviation of Burn-Induced Intestinal Injury via Activating YAP: A Preliminary Study
Abstract
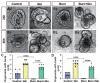
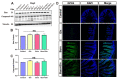
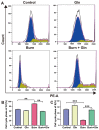
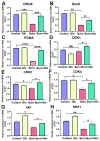
Burn injury is a common form of traumatic injury that leads to high mortality worldwide. A severe burn injury usually induces gut barrier dysfunction, partially resulting from the impairment in the proliferation and self-renewal of intestinal stem cells (ISCs) post burns. As a main energy substance of small intestinal enterocytes, glutamine (Gln) is important for intestinal cell viability and growth, while its roles in ISCs-induced regeneration after burns are still unclear. To demonstrate the potential effects of Gln in improving ISCs proliferation and alleviating burn-induced intestinal injury, in this study, we verified that Gln significantly alleviated small intestine injury in burned mice model. It showed that Gln could significantly decrease the ferroptosis of crypt cells in the ileum, promote the proliferation of ISCs, and repair the crypt. These effects of Gln were also confirmed in the mouse small intestine organoids model. Further research found that Yes-associated protein (YAP) is suppressed after burn injury, and Gln could improve cell proliferation and accelerate the renewal of the damaged intestinal mucosal barrier after burns by activating YAP. YAP is closely associated with the changes in intestinal stem cell proliferation after burn injury and could be served as a potential target for severe burns.
Keywords: burns; crypt; glutamine; intestinal stem cells; proliferation.
Conflict of interest statement
The authors declare no competing interest.
Figures



Similar articles
-
Glutamine promotes the proliferation of intestinal stem cells via inhibition of TP53-induced glycolysis and apoptosis regulator promoter methylation in burned mice.Burns Trauma. 2024 Sep 26;12:tkae045. doi: 10.1093/burnst/tkae045. eCollection 2024. Burns Trauma. 2024. PMID: 39328365 Free PMC article.
-
Influence of Growth Hormone and Glutamine on Intestinal Stem Cells: A Narrative Review.Nutrients. 2019 Aug 17;11(8):1941. doi: 10.3390/nu11081941. Nutrients. 2019. PMID: 31426533 Free PMC article. Review.
-
Effect of parenteral glutamine supplementation combined with enteral nutrition on Hsp90 expression and Peyer's patch apoptosis in severely burned rats.Nutrition. 2018 Mar;47:97-103. doi: 10.1016/j.nut.2017.10.005. Epub 2018 Jan 4. Nutrition. 2018. PMID: 29429543
-
Distinct Effects of Growth Hormone and Glutamine on Activation of Intestinal Stem Cells.JPEN J Parenter Enteral Nutr. 2018 Mar;42(3):642-651. doi: 10.1177/0148607117709435. Epub 2017 Dec 19. JPEN J Parenter Enteral Nutr. 2018. PMID: 28510488
-
The Hippo-YAP/TAZ Signaling Pathway in Intestinal Self-Renewal and Regeneration After Injury.Front Cell Dev Biol. 2022 Jul 19;10:894737. doi: 10.3389/fcell.2022.894737. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35927987 Free PMC article. Review.
Cited by
-
Metabolic Control of Stem Cell Function: Insights into Amino Acid-Driven Mechanisms.Stem Cell Rev Rep. 2025 Sep 5. doi: 10.1007/s12015-025-10967-9. Online ahead of print. Stem Cell Rev Rep. 2025. PMID: 40911162 Review.
-
Glutamine promotes the proliferation of intestinal stem cells via inhibition of TP53-induced glycolysis and apoptosis regulator promoter methylation in burned mice.Burns Trauma. 2024 Sep 26;12:tkae045. doi: 10.1093/burnst/tkae045. eCollection 2024. Burns Trauma. 2024. PMID: 39328365 Free PMC article.
-
Glutamine Regulates Gene Expression Profiles to Increase the Proliferation of Porcine Intestinal Epithelial Cells and the Expansion of Intestinal Stem Cells.Animals (Basel). 2023 Sep 14;13(18):2917. doi: 10.3390/ani13182917. Animals (Basel). 2023. PMID: 37760316 Free PMC article.
-
Metabolic regulation of intestinal homeostasis: molecular and cellular mechanisms and diseases.MedComm (2020). 2024 Oct 25;5(11):e776. doi: 10.1002/mco2.776. eCollection 2024 Nov. MedComm (2020). 2024. PMID: 39465140 Free PMC article. Review.
-
IGFBP7 promotes endothelial cell repair in the recovery phase of acute lung injury.Clin Sci (Lond). 2024 Jul 3;138(13):797-815. doi: 10.1042/CS20240179. Clin Sci (Lond). 2024. PMID: 38840498 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

