Mutated Isocitrate Dehydrogenase (mIDH) as Target for PET Imaging in Gliomas
- PMID: 37049661
- PMCID: PMC10096429
- DOI: 10.3390/molecules28072890
Mutated Isocitrate Dehydrogenase (mIDH) as Target for PET Imaging in Gliomas
Abstract
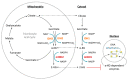
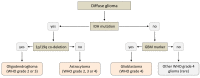
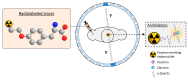
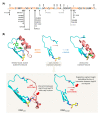
Gliomas are the most common primary brain tumors in adults. A diffuse infiltrative growth pattern and high resistance to therapy make them largely incurable, but there are significant differences in the prognosis of patients with different subtypes of glioma. Mutations in isocitrate dehydrogenase (IDH) have been recognized as an important biomarker for glioma classification and a potential therapeutic target. However, current clinical methods for detecting mutated IDH (mIDH) require invasive tissue sampling and cannot be used for follow-up examinations or longitudinal studies. PET imaging could be a promising approach for non-invasive assessment of the IDH status in gliomas, owing to the availability of various mIDH-selective inhibitors as potential leads for the development of PET tracers. In the present review, we summarize the rationale for the development of mIDH-selective PET probes, describe their potential applications beyond the assessment of the IDH status and highlight potential challenges that may complicate tracer development. In addition, we compile the major chemical classes of mIDH-selective inhibitors that have been described to date and briefly consider possible strategies for radiolabeling of the most promising candidates. Where available, we also summarize previous studies with radiolabeled analogs of mIDH inhibitors and assess their suitability for PET imaging in gliomas.
Keywords: fluorine-18; glioma; molecular imaging; mutated isocitrate dehydrogenase (mIDH); positron emission tomography (PET); radiotracer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

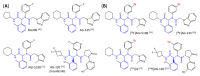
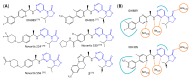
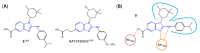
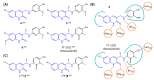
Similar articles
-
Preparation and Preclinical Evaluation of 18F-Labeled Olutasidenib Derivatives for Non-Invasive Detection of Mutated Isocitrate Dehydrogenase 1 (mIDH1).Molecules. 2024 Aug 21;29(16):3939. doi: 10.3390/molecules29163939. Molecules. 2024. PMID: 39203017 Free PMC article.
-
Static 18F-FET PET and DSC-PWI based on hybrid PET/MR for the prediction of gliomas defined by IDH and 1p/19q status.Eur Radiol. 2021 Jun;31(6):4087-4096. doi: 10.1007/s00330-020-07470-9. Epub 2020 Nov 19. Eur Radiol. 2021. PMID: 33211141
-
Potential of IDH mutations as immunotherapeutic targets in gliomas: a review and meta-analysis.Expert Opin Ther Targets. 2021 Dec;25(12):1045-1060. doi: 10.1080/14728222.2021.2017422. Expert Opin Ther Targets. 2021. PMID: 34904924 Review.
-
Clinical applications and prospects of PET imaging in patients with IDH-mutant gliomas.J Neurooncol. 2023 May;162(3):481-488. doi: 10.1007/s11060-022-04218-x. Epub 2022 Dec 29. J Neurooncol. 2023. PMID: 36577872 Free PMC article. Review.
-
Noninvasive assessment of isocitrate dehydrogenase mutation status in cerebral gliomas by magnetic resonance spectroscopy in a clinical setting.J Neurosurg. 2018 Feb;128(2):391-398. doi: 10.3171/2016.10.JNS161793. Epub 2017 Mar 3. J Neurosurg. 2018. PMID: 28298040
Cited by
-
PET imaging of gliomas: Status quo and quo vadis?Neuro Oncol. 2024 Dec 9;26(Supplement_9):S185-S198. doi: 10.1093/neuonc/noae078. Neuro Oncol. 2024. PMID: 38970818 Free PMC article. Review.
-
From Inhibitors to PET: SAR-Based Development of [18F]SK60 for mIDH1 Imaging.J Med Chem. 2025 Jul 10;68(13):13750-13771. doi: 10.1021/acs.jmedchem.5c00584. Epub 2025 Jun 29. J Med Chem. 2025. PMID: 40581853 Free PMC article.
-
Radiosynthesis and biological evaluation of [18F]AG-120 for PET imaging of the mutant isocitrate dehydrogenase 1 in glioma.Eur J Nucl Med Mol Imaging. 2024 Mar;51(4):1085-1096. doi: 10.1007/s00259-023-06515-7. Epub 2023 Nov 20. Eur J Nucl Med Mol Imaging. 2024. PMID: 37982850 Free PMC article.
-
Preparation and Preclinical Evaluation of 18F-Labeled Olutasidenib Derivatives for Non-Invasive Detection of Mutated Isocitrate Dehydrogenase 1 (mIDH1).Molecules. 2024 Aug 21;29(16):3939. doi: 10.3390/molecules29163939. Molecules. 2024. PMID: 39203017 Free PMC article.
-
Beyond invasive biopsies: using VASARI MRI features to predict grade and molecular parameters in gliomas.Cancer Imaging. 2024 Jan 2;24(1):3. doi: 10.1186/s40644-023-00638-8. Cancer Imaging. 2024. PMID: 38167551 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

