Globally Accurate Gaussian Process Potential Energy Surface and Quantum Dynamics Studies on the Li(2S) + Na2 → LiNa + Na Reaction at Low Collision Energies
- PMID: 37049701
- PMCID: PMC10096016
- DOI: 10.3390/molecules28072938
Globally Accurate Gaussian Process Potential Energy Surface and Quantum Dynamics Studies on the Li(2S) + Na2 → LiNa + Na Reaction at Low Collision Energies
Abstract
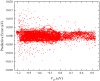
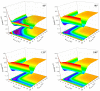
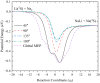
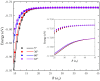
The LiNa2 reactive system has recently received great attention in the experimental study of ultracold chemical reactions, but the corresponding theoretical calculations have not been carried out. Here, we report the first globally accurate ground-state LiNa2 potential energy surface (PES) using a Gaussian process model based on only 1776 actively selected high-level ab initio training points. The constructed PES had high precision and strong generalization capability. On the new PES, the quantum dynamics calculations on the Li(2S) + Na2(v = 0, j = 0) → LiNa + Na reaction were carried out in the 0.001-0.01 eV collision energy range using an improved time-dependent wave packet method. The calculated results indicate that this reaction is dominated by a complex-forming mechanism at low collision energies. The presented dynamics data provide guidance for experimental research, and the newly constructed PES could be further used for ultracold reaction dynamics calculations on this reactive system.
Keywords: Gaussian process; ab initio; potential energy surface; reaction dynamics; time-dependent wave packet.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
A Globally Accurate Neural Network Potential Energy Surface and Quantum Dynamics Studies on Be+(2S) + H2/D2 → BeH+/BeD+ + H/D Reactions.Molecules. 2024 Jul 22;29(14):3436. doi: 10.3390/molecules29143436. Molecules. 2024. PMID: 39065017 Free PMC article.
-
A neural network potential energy surface for the Li + LiNa → Li2 + Na reaction and quantum dynamics study from ultracold to thermal energies.Phys Chem Chem Phys. 2023 Jul 19;25(28):19024-19036. doi: 10.1039/d3cp01753b. Phys Chem Chem Phys. 2023. PMID: 37417472
-
Quantum Dynamics Studies of the Li + Na2 (V = 0, j = 0) → Na + NaLi Reaction on a New Neural Network Potential Energy Surface.J Phys Chem A. 2024 Jul 4;128(26):5115-5127. doi: 10.1021/acs.jpca.4c01891. Epub 2024 Jun 18. J Phys Chem A. 2024. PMID: 38889710
-
Ab Initio Neural Network Potential Energy Surface and Quantum Dynamics Calculations on Na(2S) + H2 → NaH + H Reaction.Molecules. 2024 Oct 14;29(20):4871. doi: 10.3390/molecules29204871. Molecules. 2024. PMID: 39459240 Free PMC article.
-
A neural network potential energy surface and quantum dynamics studies for the Ca+(2S) + H2 → CaH+ + H reaction.Phys Chem Chem Phys. 2022 Aug 17;24(32):19209-19217. doi: 10.1039/d2cp02711a. Phys Chem Chem Phys. 2022. PMID: 35920167
Cited by
-
State-to-State Quantum Dynamics Study of Intramolecular Isotope Effects on Be(1S) + HD (v0 = 2, j0 = 0) → BeH/BeD + H/D Reaction.Molecules. 2024 Mar 13;29(6):1263. doi: 10.3390/molecules29061263. Molecules. 2024. PMID: 38542900 Free PMC article.
-
A Globally Accurate Neural Network Potential Energy Surface and Quantum Dynamics Studies on Be+(2S) + H2/D2 → BeH+/BeD+ + H/D Reactions.Molecules. 2024 Jul 22;29(14):3436. doi: 10.3390/molecules29143436. Molecules. 2024. PMID: 39065017 Free PMC article.
References
-
- Ospelkaus S., Pe’er A., Ni K.K., Zirbel J.J., Neyenhuis B., Kotochigova S., Julienne P.S., Ye J., Jin D.S. Efficient state transfer in an ultracold dense gas of heteronuclear molecules. Nat. Phys. 2008;4:622–626. doi: 10.1038/nphys997. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

