Evaluation of Selenomethionine Entrapped in Nanoparticles for Oral Supplementation Using In Vitro, Ex Vivo and In Vivo Models
- PMID: 37049704
- PMCID: PMC10095941
- DOI: 10.3390/molecules28072941
Evaluation of Selenomethionine Entrapped in Nanoparticles for Oral Supplementation Using In Vitro, Ex Vivo and In Vivo Models
Abstract
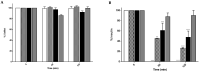
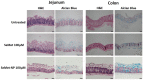
Selenium methionine (SeMet) is an essential micronutrient required for normal body function and is associated with additional health benefits. However, oral administration of SeMet can be challenging due to its purported narrow therapeutic index, low oral bioavailability, and high susceptibility to oxidation. To address these issues, SeMet was entrapped in zein-coated nanoparticles made from chitosan using an ionic gelation formulation. The high stability of both the SeMet and selenomethionine nanoparticles (SeMet-NPs) was established using cultured human intestinal and liver epithelial cells, rat liver homogenates, and rat intestinal homogenates and lumen washes. Minimal cytotoxicity to Caco-2 and HepG2 cells was observed for SeMet and SeMet-NPs. Antioxidant properties of SeMet were revealed using a Reactive Oxygen Species (ROS) assay, based on the observation of a concentration-dependent reduction in the build-up of peroxides, hydroxides and hydroxyl radicals in Caco-2 cells exposed to SeMet (6.25-100 μM). The basal apparent permeability coefficient (Papp) of SeMet across isolated rat jejunal mucosae mounted in Ussing chambers was low, but the Papp was increased when presented in NP. SeMet had minimal effects on the electrogenic ion secretion of rat jejunal and colonic mucosae in Ussing chambers. Intra-jejunal injections of SeMet-NPs to rats yielded increased plasma levels of SeMet after 3 h for the SeMet-NPs compared to free SeMet. Overall, there is potential to further develop SeMet-NPs for oral supplementation due to the increased intestinal permeability, versus free SeMet, and the low potential for toxicity.
Keywords: cytotoxicity; intestinal drug transport; nanoparticles; nutraceuticals; oral delivery of micronutrients; selenium methionine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Application of Box-Behnken experimental design for the formulation and optimisation of selenomethionine-loaded chitosan nanoparticles coated with zein for oral delivery.Int J Pharm. 2018 Nov 15;551(1-2):257-269. doi: 10.1016/j.ijpharm.2018.08.050. Epub 2018 Aug 25. Int J Pharm. 2018. PMID: 30153488
-
Selenium Kinetics in Humans Change Following 2 Years of Supplementation With Selenomethionine.Front Endocrinol (Lausanne). 2021 Mar 29;12:621687. doi: 10.3389/fendo.2021.621687. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33859616 Free PMC article.
-
Selenomethionine protects hematopoietic stem/progenitor cells against cobalt nanoparticles by stimulating antioxidant actions and DNA repair functions.Aging (Albany NY). 2021 Apr 19;13(8):11705-11726. doi: 10.18632/aging.202865. Epub 2021 Apr 19. Aging (Albany NY). 2021. PMID: 33875618 Free PMC article.
-
The nutritional significance, metabolism and toxicology of selenomethionine.Adv Food Nutr Res. 2003;47:73-112. doi: 10.1016/s1043-4526(03)47002-2. Adv Food Nutr Res. 2003. PMID: 14639782 Review.
-
Selenomethionine: A Pink Trojan Redox Horse with Implications in Aging and Various Age-Related Diseases.Antioxidants (Basel). 2021 May 31;10(6):882. doi: 10.3390/antiox10060882. Antioxidants (Basel). 2021. PMID: 34072794 Free PMC article. Review.
References
-
- Aderao G.N., Jadhav S.E., Pattanaik A.K., Gupta S.K., Ramakrishnan S., Lokesha E., Chaudhary P., Vaswani S., Singh A., Panigrahi M., et al. Dietary selenium levels modulates antioxidant, cytokine and immune response and selenoproteins mRNA expression in rats under heat stress condition. J. Trace Elem. Med. Biol. 2023;75:127105. doi: 10.1016/j.jtemb.2022.127105. - DOI - PubMed
-
- Gutierrez-Bedmar M., Gil F., Olmedo P., Ruiz-Canela M., Martinez-Gonzalez M.A., Salas-Salvado J., Babio N., Fito M., Del Val Garcia J.L., Corella D., et al. Serum Selenium and Incident Cardiovascular Disease in the PREvencion con DIeta MEDiterranea (PREDIMED) Trial: Nested Case-Control Study. J. Clin. Med. 2022;11:6664. doi: 10.3390/jcm11226664. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

