An Efficient Continuous Flow Synthesis for the Preparation of N-Arylhydroxylamines: Via a DMAP-Mediated Hydrogenation Process
- PMID: 37049731
- PMCID: PMC10096002
- DOI: 10.3390/molecules28072968
An Efficient Continuous Flow Synthesis for the Preparation of N-Arylhydroxylamines: Via a DMAP-Mediated Hydrogenation Process
Abstract
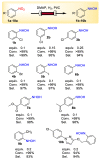
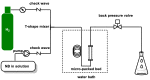
The selective hydrogenation of nitroarenes to N-arylhydroxylamines is an important synthetic process in the chemical industry. It is commonly accomplished by using heterogeneous catalytic systems that contain inhibitors, such as DMSO. Herein, DMAP has been identified as a unique additive for increasing hydrogenation activity and product selectivity (up to >99%) under mild conditions in the Pt/C-catalyzed process. Continuous-flow technology has been explored as an efficient approach toward achieving the selective hydrogenation of nitroarenes to N-arylhydroxylamines. The present flow protocol was applied for a vast substrate scope and was found to be compatible with a wide range of functional groups, such as electron-donating groups, carbonyl, and various halogens. Further studies were attempted to show that the improvement in the catalytic activity and selectivity benefited from the dual functions of DMAP; namely, the heterolytic H2 cleavage and competitive adsorption.
Keywords: 4-(dimethylamino) pyridine; N-arylhydroxylamine; catalytic activity; continuous flow; selectivity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






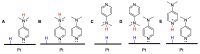



Similar articles
-
Carbon supported Pt colloid as effective catalyst for selective hydrogenation of nitroarenes to arylhydroxylamines.Chem Commun (Camb). 2010 Mar 7;46(9):1559-61. doi: 10.1039/b916686f. Epub 2010 Jan 8. Chem Commun (Camb). 2010. PMID: 20162181
-
Selective hydrogenation of nitroaromatics to N-arylhydroxylamines in a micropacked bed reactor with passivated catalyst.RSC Adv. 2020 Aug 3;10(48):28585-28594. doi: 10.1039/d0ra05715k. eCollection 2020 Aug 3. RSC Adv. 2020. PMID: 35520060 Free PMC article.
-
A high activity mesoporous Pt@KIT-6 nanocomposite for selective hydrogenation of halogenated nitroarenes in a continuous-flow microreactor.Nanoscale Adv. 2023 Sep 14;5(20):5649-5660. doi: 10.1039/d3na00437f. eCollection 2023 Oct 10. Nanoscale Adv. 2023. PMID: 37822898 Free PMC article.
-
Heterogeneous catalytic hydrogenation reactions in continuous-flow reactors.ChemSusChem. 2011 Mar 21;4(3):300-16. doi: 10.1002/cssc.201000354. Epub 2011 Feb 17. ChemSusChem. 2011. PMID: 21337528 Review.
-
Recent Progress in Pd-Based Nanocatalysts for Selective Hydrogenation.ACS Omega. 2021 Dec 20;7(1):17-31. doi: 10.1021/acsomega.1c06244. eCollection 2022 Jan 11. ACS Omega. 2021. PMID: 35036674 Free PMC article. Review.
Cited by
-
Catalytic Reduction of Aromatic Nitro Compounds to Phenylhydroxylamine and Its Derivatives.Molecules. 2024 Sep 13;29(18):4353. doi: 10.3390/molecules29184353. Molecules. 2024. PMID: 39339349 Free PMC article. Review.
References
-
- Yuan H., Guo L., Liu F., Miao Z., Feng L., Gao H. Copper-catalyzed tandem o-vinylation of arylhydroxylamines/[3,3]-rearrangement/cyclization: Synthesis of highly substituted indoles and benzoindoles. ACS Catal. 2019;9:3906–3912. doi: 10.1021/acscatal.9b00470. - DOI
-
- Dounay A.B., Anderson M., Bechle B.M., Campbell B.M., Claffey M.M., Evdokimov A., Evrard E., Fonseca K.R., Gan X., Ghosh S., et al. Discovery of brain-penetrant, irreversible kynurenine aminotransferase ii inhibitors for schizophrenia. ACS Med. Chem. Lett. 2012;3:187–192. doi: 10.1021/ml200204m. - DOI - PMC - PubMed
-
- Vyas P.M., Roychowdhury S., Woster P.M., Svensson C.K. Reactive oxygen species generation and its role in the differential cytotoxicity of the arylhydroxylamine metabolites of sulfamethoxazole and dapsone in normal human epidermal keratinocytes. Biochem. Pharmacol. 2005;70:275–286. doi: 10.1016/j.bcp.2005.04.023. - DOI - PubMed
-
- Yadav J.S., Reddy B.V.S., Sreedhar P. Three-component one-pot synthesis of α-hydroxylamino phosphonates using ionic liquids. Adv. Synth. Catal. 2003;345:564–567. doi: 10.1002/adsc.200202209. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

