Phytochemical Profile and Antimicrobial Potential of Propolis Samples from Kazakhstan
- PMID: 37049747
- PMCID: PMC10095981
- DOI: 10.3390/molecules28072984
Phytochemical Profile and Antimicrobial Potential of Propolis Samples from Kazakhstan
Abstract
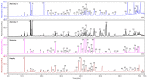
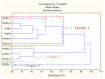
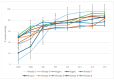
In the current paper, we present the results of Kazakh propolis investigations. Due to limited data about propolis from this country, research was focused mainly on phytochemical analysis and evaluation of propolis antimicrobial activity. uHPLC-DAD (ultra-high-pressure-liquid chromatography coupled with diode array detection, UV/VIS) and uHPLC-MS/MS (ultra-high-pressure-liquid chromatography coupled with tandem mass spectrometry) were used to phytochemical characteristics while antimicrobial activity was evaluated in the serial dilution method (MIC, minimal inhibitory concentration, and MBC/MFC, minimal bactericidal/fungicidal concentration measurements). In the study, Kazakh propolis exhibited a strong presence of markers characteristic of poplar-type propolis-flavonoid aglycones (pinocembrin, galangin, pinobanksin and pinobanskin-3-O-acetate) and hydroxycinnamic acid monoesters (mainly caffeic acid phenethyl ester and different isomers of caffeic acid prenyl ester). The second plant precursor of Kazakh propolis was aspen-poplar with 2-acetyl-1,3-di-p-coumaroyl glycerol as the main marker. Regarding antimicrobial activity, Kazakh propolis revealed stronger activity against reference Gram-positive strains (MIC from 31.3 to above 4000 mg/L) and yeasts (MIC from 62.5 to 1000 mg/L) than against reference Gram-negative strains (MIC ≥ 4000 mg/L). Moreover, Kazakh propolis showed good anti-Helicobacter pylori activity (MIC and MBC were from 31.3 to 62.5 mg/L). All propolis samples were also tested for H. pylori urease inhibitory activity (IC50, half-maximal inhibitory concentration, ranged from 440.73 to 11,177.24 µg/mL). In summary Kazakh propolis are potent antimicrobial agents and may be considered as a medicament in the future.
Keywords: Helicobacter pylori; Kazakhstan; black poplar; dendrogram; flavonoids antibacterial; hydroethanolic extracts; propolis; urease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Correlation between Chemical Profile of Georgian Propolis Extracts and Their Activity against Helicobacter pylori.Molecules. 2023 Feb 1;28(3):1374. doi: 10.3390/molecules28031374. Molecules. 2023. PMID: 36771040 Free PMC article.
-
Phytochemical Profile, Plant Precursors and Some Properties of Georgian Propolis.Molecules. 2022 Nov 9;27(22):7714. doi: 10.3390/molecules27227714. Molecules. 2022. PMID: 36431810 Free PMC article.
-
The Antimicrobial Properties of Poplar and Aspen-Poplar Propolises and Their Active Components against Selected Microorganisms, including Helicobacter pylori.Pathogens. 2022 Jan 31;11(2):191. doi: 10.3390/pathogens11020191. Pathogens. 2022. PMID: 35215134 Free PMC article.
-
Phytochemical Profiles and Antimicrobial Activity of Selected Populus spp. Bud Extracts.Molecules. 2024 Jan 16;29(2):437. doi: 10.3390/molecules29020437. Molecules. 2024. PMID: 38257349 Free PMC article.
-
Growth Biocontrol of Foodborne Pathogens and Spoilage Microorganisms of Food by Polish Propolis Extracts.Molecules. 2019 Aug 15;24(16):2965. doi: 10.3390/molecules24162965. Molecules. 2019. PMID: 31443325 Free PMC article.
Cited by
-
Exploring the Functional Properties of Propolis, Geopropolis, and Cerumen, with a Special Emphasis on Their Antimicrobial Effects.Foods. 2023 Oct 25;12(21):3909. doi: 10.3390/foods12213909. Foods. 2023. PMID: 37959028 Free PMC article. Review.
-
Bioactive Compounds from Propolis on Bone Homeostasis: A Narrative Review.Antioxidants (Basel). 2025 Jan 12;14(1):81. doi: 10.3390/antiox14010081. Antioxidants (Basel). 2025. PMID: 39857415 Free PMC article. Review.
-
Evaluation of the Chemical Profile and Antioxidant Capacity of Green, Brown, and Dark Propolis.Plants (Basel). 2023 Sep 8;12(18):3204. doi: 10.3390/plants12183204. Plants (Basel). 2023. PMID: 37765368 Free PMC article.
-
In Vitro Antimicrobial Potential of Portuguese Propolis Extracts from Gerês against Pathogenic Microorganisms.Antibiotics (Basel). 2024 Jul 16;13(7):655. doi: 10.3390/antibiotics13070655. Antibiotics (Basel). 2024. PMID: 39061337 Free PMC article.
-
Screening the Extract of Laportea bulbifera (Sieb. et Zucc.) Wedd. Based on Active Component Content, Its Antioxidant Capacity and Exploration of Hepatoprotective Activity in Rats.Molecules. 2023 Aug 25;28(17):6256. doi: 10.3390/molecules28176256. Molecules. 2023. PMID: 37687084 Free PMC article.
References
-
- Regueira M.S., Tintino S.R., da Silva A.R.P., Costa M.D.S., Boligon A.A., Matias E.F.F., de Queiroz Balbino V., Menezes I.R.A., Melo Coutinho H.D. Seasonal variation of Brazilian red propolis: Antibacterial activity, synergistic effect and phytochemical screening. Food Chem. Toxicol. 2017;107:572–580. doi: 10.1016/j.fct.2017.03.052. - DOI - PubMed
-
- Da Silva R.O., Andrade V.M., Bullé Rêgo E.S., Azevedo Dória G.A., Santos Lima B.D., da Silva F.A., de Souza Araújo A.A., de Albuquerque Júnior R.L.C., Cordeiro Cardoso J., Zanardo Gomes M. Acute and sub-acute oral toxicity of Brazilian red propolis in rats. J. Ethnopharmacol. 2015;170:66–71. doi: 10.1016/j.jep.2015.05.009. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

