Amphiphilic Cyclodextrin Nanoparticles as Delivery System for Idebenone: A Preformulation Study
- PMID: 37049785
- PMCID: PMC10096402
- DOI: 10.3390/molecules28073023
Amphiphilic Cyclodextrin Nanoparticles as Delivery System for Idebenone: A Preformulation Study
Abstract
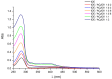
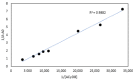
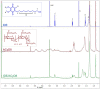
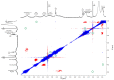
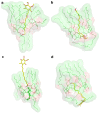
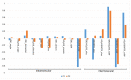
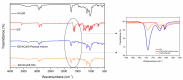
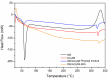
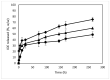
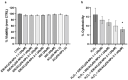
Idebenone (IDE), a synthetic short-chain analogue of coenzyme Q10, is a potent antioxidant able to prevent lipid peroxidation and stimulate nerve growth factor. Due to these properties, IDE could potentially be active towards cerebral disorders, but its poor water solubility limits its clinical application. Octanoyl-β-cyclodextrin is an amphiphilic cyclodextrin (ACyD8) bearing, on average, ten octanoyl substituents able to self-assemble in aqueous solutions, forming various typologies of supramolecular nanoassemblies. Here, we developed nanoparticles based on ACyD8 (ACyD8-NPs) for the potential intranasal administration of IDE to treat neurological disorders, such as Alzheimer's Disease. Nanoparticles were prepared using the nanoprecipitation method and were characterized for their size, zeta potential and morphology. STEM images showed spherical particles, with smooth surfaces and sizes of about 100 nm, suitable for the proposed therapeutical aim. The ACyD8-NPs effectively loaded IDE, showing a high encapsulation efficiency and drug loading percentage. To evaluate the host/guest interaction, UV-vis titration, mono- and two-dimensional NMR analyses, and molecular modeling studies were performed. IDE showed a high affinity for the ACyD8 cavity, forming a 1:1 inclusion complex with a high association constant. A biphasic and sustained release of IDE was observed from the ACyD8-NPs, and, after a burst effect of about 40%, the release was prolonged over 10 days. In vitro studies confirmed the lack of toxicity of the IDE/ACyD8-NPs on neuronal SH-SY5Y cells, and they demonstrated their antioxidant effect upon H2O2 exposure, as a general source of ROS.
Keywords: NMR studies; amphiphilic cyclodextrins; idebenone; in vitro antioxidant activity; molecular modeling; nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Chitosan/Cyclodextrin Nanospheres for Potential Nose-to-Brain Targeting of Idebenone.Pharmaceuticals (Basel). 2022 Sep 28;15(10):1206. doi: 10.3390/ph15101206. Pharmaceuticals (Basel). 2022. PMID: 36297318 Free PMC article.
-
Randomly methylated β-cyclodextrin improves water - solubility, cellular protection and mucosa permeability of idebenone.Int J Pharm. 2024 Nov 15;665:124718. doi: 10.1016/j.ijpharm.2024.124718. Epub 2024 Sep 15. Int J Pharm. 2024. PMID: 39288841
-
In Vitro Antioxidant Activity of Idebenone Derivative-Loaded Solid Lipid Nanoparticles.Molecules. 2017 May 27;22(6):887. doi: 10.3390/molecules22060887. Molecules. 2017. PMID: 28555014 Free PMC article.
-
Idebenone: Novel Strategies to Improve Its Systemic and Local Efficacy.Nanomaterials (Basel). 2018 Feb 5;8(2):87. doi: 10.3390/nano8020087. Nanomaterials (Basel). 2018. PMID: 29401722 Free PMC article. Review.
-
[Amphiphilic cyclodextrins and their applications. Preparation of nanoparticles based on amphiphilic cyclodextrins for biomedical applications].Ann Pharm Fr. 2010 Jan;68(1):12-26. doi: 10.1016/j.pharma.2009.12.002. Epub 2010 Feb 6. Ann Pharm Fr. 2010. PMID: 20176159 Review. French.
Cited by
-
Characterization and In Vivo Antiangiogenic Activity Evaluation of Morin-Based Cyclodextrin Inclusion Complexes.Pharmaceutics. 2023 Aug 26;15(9):2209. doi: 10.3390/pharmaceutics15092209. Pharmaceutics. 2023. PMID: 37765179 Free PMC article.
-
Host-Guest Interaction Study of Olmesartan Medoxomil with β-Cyclodextrin Derivatives.Molecules. 2024 May 8;29(10):2209. doi: 10.3390/molecules29102209. Molecules. 2024. PMID: 38792072 Free PMC article.
-
The Anti-Angiogenic Effect of Cynara cardunculus L. subsp. cardunculus Waste Product.Foods. 2025 Jul 29;14(15):2656. doi: 10.3390/foods14152656. Foods. 2025. PMID: 40807592 Free PMC article.
-
Host-Guest Complexation of Olmesartan Medoxomil by Heptakis(2,6-di-O-methyl)-β-cyclodextrin: Compatibility Study with Excipients.Pharmaceutics. 2024 Dec 4;16(12):1557. doi: 10.3390/pharmaceutics16121557. Pharmaceutics. 2024. PMID: 39771536 Free PMC article.
-
Naringenin-Loaded Solid Lipid Nanoparticles: Physical-Chemical Characterization and In Vitro Antibacterial Activity.Pharmaceuticals (Basel). 2025 Feb 8;18(2):232. doi: 10.3390/ph18020232. Pharmaceuticals (Basel). 2025. PMID: 40006044 Free PMC article.
References
-
- Lin P., Liu J., Ren M., Ji K., Li L., Zhang B., Gong Y., Yan C. Idebenone Protects against Oxidized Low Density Lipoprotein Induced Mitochondrial Dysfunction in Vascular Endothelial Cells via GSK3β/β-Catenin Signalling Pathways. Biochem. Biophys. Res. Commun. 2015;465:548–555. doi: 10.1016/j.bbrc.2015.08.058. - DOI - PubMed
-
- Erb M., Hoffmann-Enger B., Deppe H., Soeberdt M., Haefeli R.H., Rummey C., Feurer A., Gueven N. Features of Idebenone and Related Short-Chain Quinones That Rescue ATP Levels under Conditions of Impaired Mitochondrial Complex I. PLoS ONE. 2012;7:e36153. doi: 10.1371/journal.pone.0036153. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

