Activation-Free Sulfonyl Fluoride Probes for Fragment Screening
- PMID: 37049805
- PMCID: PMC10096327
- DOI: 10.3390/molecules28073042
Activation-Free Sulfonyl Fluoride Probes for Fragment Screening
Abstract
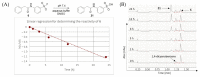
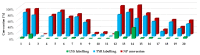
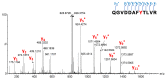
SuFEx chemistry is based on the unique reactivity of the sulfonyl fluoride group with a range of nucleophiles. Accordingly, sulfonyl fluorides label multiple nucleophilic amino acid residues, making these reagents popular in both chemical biology and medicinal chemistry applications. The reactivity of sulfonyl fluorides nominates this warhead chemotype as a candidate for an external, activation-free general labelling tag. Here, we report the synthesis and characterization of a small sulfonyl fluoride library that yielded the 3-carboxybenzenesulfonyl fluoride warhead for tagging tractable targets at nucleophilic residues. Based on these results, we propose that coupling diverse fragments to this warhead would result in a library of sulfonyl fluoride bits (SuFBits), available for screening against protein targets. SuFBits will label the target if it binds to the core fragment, which facilitates the identification of weak fragments by mass spectrometry.
Keywords: chemical probe; covalent fragment; electrophilic warhead; fragment screening; sulfonyl fluoride; targeted covalent inhibitor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Potential peptidic proteasome inhibitors by incorporation of an electrophilic trap based on amino acid derived α-substituted sulfonyl fluorides.Bioorg Med Chem. 2017 Oct 1;25(19):5055-5063. doi: 10.1016/j.bmc.2017.07.019. Epub 2017 Jul 12. Bioorg Med Chem. 2017. PMID: 28734665
-
Covalent drug discovery using sulfur(VI) fluoride exchange warheads.Expert Opin Drug Discov. 2023 Jul;18(7):725-735. doi: 10.1080/17460441.2023.2218642. Epub 2023 May 27. Expert Opin Drug Discov. 2023. PMID: 37243622 Review.
-
Proximity-enhanced SuFEx chemical cross-linker for specific and multitargeting cross-linking mass spectrometry.Proc Natl Acad Sci U S A. 2018 Oct 30;115(44):11162-11167. doi: 10.1073/pnas.1813574115. Epub 2018 Oct 15. Proc Natl Acad Sci U S A. 2018. PMID: 30322930 Free PMC article.
-
A Heck-Matsuda Process for the Synthesis of β-Arylethenesulfonyl Fluorides: Selectively Addressable Bis-electrophiles for SuFEx Click Chemistry.Angew Chem Int Ed Engl. 2016 Nov 2;55(45):14155-14158. doi: 10.1002/anie.201608807. Epub 2016 Oct 10. Angew Chem Int Ed Engl. 2016. PMID: 27723200 Free PMC article.
-
Chemical and biology of sulfur fluoride exchange (SuFEx) click chemistry for drug discovery.Bioorg Chem. 2023 Jan;130:106227. doi: 10.1016/j.bioorg.2022.106227. Epub 2022 Oct 30. Bioorg Chem. 2023. PMID: 36368173 Review.
Cited by
-
Visible-light-driven reactions for the synthesis of sulfur dioxide-inserted compounds: generation of S-F, S-O, and S-N bonds.RSC Adv. 2023 May 11;13(21):14412-14434. doi: 10.1039/d3ra02067c. eCollection 2023 May 9. RSC Adv. 2023. PMID: 37180001 Free PMC article. Review.
References
-
- Abdul Fattah T., Saeed A., Albericio F. Recent Advances towards Sulfur (VI) Fluoride Exchange (SuFEx) Click Chemistry. J. Fluor. Chem. 2018;213:87–112. doi: 10.1016/j.jfluchem.2018.07.008. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

