Preparation of Cellulose Nanocrystals by Synergistic Action of Ionic Liquid and Recyclable Solid Acid under Mild Conditions
- PMID: 37049833
- PMCID: PMC10096307
- DOI: 10.3390/molecules28073070
Preparation of Cellulose Nanocrystals by Synergistic Action of Ionic Liquid and Recyclable Solid Acid under Mild Conditions
Abstract
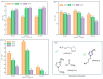
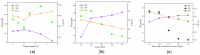
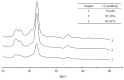
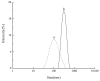
Cellulose nanocrystals (CNCs) are nanoscale particles made from cellulose. They have many unique properties such as being lightweight, stiff, and renewable, making them promising for a variety of applications in a wide range of industries, including materials science, energy storage, and biomedicine. In this paper, a two-stage (swelling-SA-catalyzed) method including IL pretreatment and solid acid hydrolysis process was developed to extract CNCs with high purity and good thermal stability from microcrystalline cellulose (MCC). In the first stage, the swelling of MCC in ionic liquid was studied with the assistance of ultrasonication, and it was found that the amorphous regions became more disordered while the crystalline areas were selectively retained under the conditions of 30 min of reaction time, 45 °C of temperature, 2% of ionic liquid water content and 1:4 mass ratio of cellulose to ionic liquid. CNCs were extracted using solid acid hydrolysis, with a 45 wt% solid acid to cellulose ratio and a 5.0 h hydrolysis process at 45 °C. The morphology, crystallinity, surface characteristics and thermo stability of the sample were characterized by atomic force microscopy (AFM), X-ray diffraction (XRD) and thermogravimetric analysis (TGA), respectively. Results demonstrated the highly thermostable CNCs were successful extracted with rodlike shape of 300 ± 100 nm in length and 20 ± 10 nm in width. Solid acid recovery and reuse were also studied, revealing a promising candidate that can reduce the environmental impact associated with chemical products.
Keywords: cellulose nanocrystals; hydrolysis; ionic liquids; recycling; solid acid; swelling.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures






Similar articles
-
Valorization of Eichhornia crassipes for the production of cellulose nanocrystals further investigation of plethoric biobased resource.Sci Rep. 2024 May 29;14(1):12387. doi: 10.1038/s41598-024-62159-z. Sci Rep. 2024. PMID: 38811644 Free PMC article.
-
Isolation and characterization of cellulose nanocrystals from pueraria root residue.Int J Biol Macromol. 2019 May 15;129:1081-1089. doi: 10.1016/j.ijbiomac.2018.07.055. Epub 2018 Aug 31. Int J Biol Macromol. 2019. PMID: 30009914
-
Morphological, Spectroscopic and Thermal Analysis of Cellulose Nanocrystals Extracted from Waste Jute Fiber by Acid Hydrolysis.Polymers (Basel). 2023 Mar 20;15(6):1530. doi: 10.3390/polym15061530. Polymers (Basel). 2023. PMID: 36987310 Free PMC article.
-
Cellulose nanocrystals from agriculture and forestry biomass: synthesis methods, characterization and industrial applications.Environ Sci Pollut Res Int. 2024 Oct;31(49):58745-58778. doi: 10.1007/s11356-024-35127-3. Epub 2024 Sep 28. Environ Sci Pollut Res Int. 2024. PMID: 39340607 Free PMC article. Review.
-
Controlling the critical parameters of ultrasonication to affect the dispersion state, isolation, and chiral nematic assembly of cellulose nanocrystals.Ultrason Sonochem. 2023 Oct;99:106581. doi: 10.1016/j.ultsonch.2023.106581. Epub 2023 Sep 3. Ultrason Sonochem. 2023. PMID: 37690260 Free PMC article. Review.
Cited by
-
Response surface optimization of ionic liquid pretreatments for maximizing cellulose nanofibril production.RSC Adv. 2023 Dec 6;13(50):35629-35638. doi: 10.1039/d3ra06930c. eCollection 2023 Nov 30. RSC Adv. 2023. PMID: 38077984 Free PMC article.
-
Environmental impact and sustainability of nanocellulose-based nitrated polymers in propellants.RSC Adv. 2025 Jul 10;15(30):24167-24191. doi: 10.1039/d5ra02169c. eCollection 2025 Jul 10. RSC Adv. 2025. PMID: 40656564 Free PMC article. Review.
-
Effect of Acid Hydrolysis Conditions on the Extraction of Cellulose Nanocrystals.Polymers (Basel). 2025 May 12;17(10):1313. doi: 10.3390/polym17101313. Polymers (Basel). 2025. PMID: 40430609 Free PMC article.
References
-
- Farooq A., Patoary M.K., Zhang M.L., Mussana H., Li M.M., Naeem M.A., Mushtaq M., Farooq A., Liu L.F. Cellulose from sources to nanocellulose and an overview of synthesis and properties of nanocellulose/zinc oxide nanocomposite materials. Int. J. Biol. Macromol. 2020;154:1050–1073. doi: 10.1016/j.ijbiomac.2020.03.163. - DOI - PubMed
-
- Mokhena T.C., John M.J. Cellulose nanomaterials: New generation materials for solving global issues. Cellulose. 2020;27:1149–1194. doi: 10.1007/s10570-019-02889-w. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

