Controllable Fabrication of Zn2+ Self-Doped TiO2 Tubular Nanocomposite for Highly Efficient Water Treatment
- PMID: 37049835
- PMCID: PMC10096178
- DOI: 10.3390/molecules28073072
Controllable Fabrication of Zn2+ Self-Doped TiO2 Tubular Nanocomposite for Highly Efficient Water Treatment
Abstract
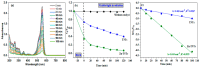
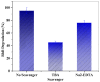
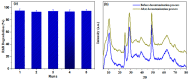
Tailoring high-efficiency photocatalytic composites for various implementations is a major research topic. 1D TNTs-based nanomaterials show promise as a photocatalyst for the remediation of organic pigments in an aqueous solution. Despite this, TiO2 (TNTs) is only photoactive in the UV range due to its inherent restriction on absorption of light in the UV range. Herein, we provide a facile recipe to tailor the optical characteristics and photocatalytic activity of TNTs by incorporating Zn (II) ionic species via an ion-exchange approach in an aqueous solution. The inclusion of Zn (II) ions into the TNTs framework expands its absorption of light toward the visible light range, therefore TiO2 nanotubes shows the visible-light photo-performance. Activity performance on photocatalytic decontamination of RhB at ambient temperature demonstrates that Zn-TNTs offer considerable boosted catalytic performance compared with untreated tubular TiO2 during the illumination of visible light. RhB (10 mg L-1) degradation of around 95% was achieved at 120 min. Radical scavenger experiment demonstrated that when electron (e-) or holes (h+) scavengers are introduced to the photodegradation process, the assessment of decontamination efficacy decreased by 45% and 76%, respectively. This demonstrates a more efficient engagement of the photoexcited electrons over photogenerated holes in the photodegradation mechanism. Furthermore, there seems to be no significant decrease in the activity of the Zn-TNTs after five consecutive runs. As a result, the fabricated Zn-TNTs composite has a high economic potential in the energy and environmental domains.
Keywords: Zn (II) ions; organic pollutants; photocatalysis; rhodamine B; titania nanotubes; visible light.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Fabrication of Ag3PO4/N-doped TiO2 nanotubes heterojunction photocatalysts for visible-light-driven photocatalysis.Chemosphere. 2024 Feb;350:141022. doi: 10.1016/j.chemosphere.2023.141022. Epub 2023 Dec 21. Chemosphere. 2024. PMID: 38141677
-
[Spectral Analysis of CdZnSe Ternary Quantum Dots Sensitized TiO2 Tubes and Its Application in Visible-Light Photocatalysis].Guang Pu Xue Yu Guang Pu Fen Xi. 2015 Nov;35(11):3161-6. Guang Pu Xue Yu Guang Pu Fen Xi. 2015. PMID: 26978928 Chinese.
-
[Fabrication and photocatalytic activity of Pt-inserted titania nanotubes].Guang Pu Xue Yu Guang Pu Fen Xi. 2009 Jun;29(6):1623-6. Guang Pu Xue Yu Guang Pu Fen Xi. 2009. PMID: 19810545 Chinese.
-
Titania nanotubes prepared by rapid breakdown anodization for photocatalytic decolorization of organic dyes under UV and natural solar light.Nanoscale Res Lett. 2018 Jun 14;13(1):179. doi: 10.1186/s11671-018-2591-5. Nanoscale Res Lett. 2018. PMID: 29900489 Free PMC article.
-
[Spectral Characteristics and Catalytic Performances of SO2-4/Ce-TiO2 with Visible Light Response].Guang Pu Xue Yu Guang Pu Fen Xi. 2016 Apr;36(4):1133-8. Guang Pu Xue Yu Guang Pu Fen Xi. 2016. PMID: 30052013 Chinese.
Cited by
-
Multidimensional insights of electrochemical and quantum investigations of morpholinium cationic surfactants as corrosion inhibitors for carbon steel in acidic solution.Sci Rep. 2025 Jun 20;15(1):20175. doi: 10.1038/s41598-025-05836-x. Sci Rep. 2025. PMID: 40542061 Free PMC article.
References
-
- Carp O., Huisman C.L., Reller A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004;32:33–177. doi: 10.1016/j.progsolidstchem.2004.08.001. - DOI
-
- Diebold U. The surface science of titanium dioxide. Surf. Sci. Rep. 2003;48:53–229. doi: 10.1016/S0167-5729(02)00100-0. - DOI
-
- Hanaor D.A., Sorell C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011;46:855–874. doi: 10.1007/s10853-010-5113-0. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

