Anticancer Properties of 3-Dietoxyphosphorylfuroquinoline-4,9-dione
- PMID: 37049894
- PMCID: PMC10095652
- DOI: 10.3390/molecules28073128
Anticancer Properties of 3-Dietoxyphosphorylfuroquinoline-4,9-dione
Abstract
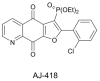
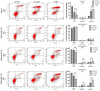
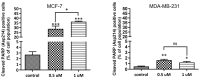
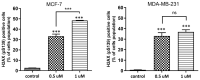
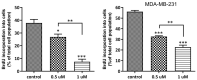
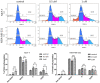
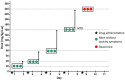
Herein, the antitumor activity of a novel synthetic analog with 5,8-quinolinedione scaffold, diethyl (2-(2-chlorophenyl)-4,9-dioxo-4,9-dihydrofuro [3,2-g]quinolin-3-yl)phosphonate (AJ-418) was investigated on two breast cancer cell lines. This analog was selected from a small library of synthetic quinolinediones on the basis of its strong antiproliferative activity against MCF-7 and MDA-MB-231 cells and 4-5-fold lower cytotoxicity towards healthy MCF-10A cells. The morphology of MCF-7 and MDA-MB-231 cancer cells treated with AJ-418 changed drastically, while non-tumorigenic MCF-10A cells remained unaffected. In MCF-7 cells, after 24 h incubation, the increased number of apoptotic cells coincided with a decrease in proliferation and cell viability. The 24 h treatment of MDA-MB-231 cells with the tested compound reduced their cell viability and proliferation rate; however, a significant pro-apoptotic effect was visible only after longer incubation times (48 h and 72 h). Then, the maximum tolerated dose (MTD) of compound AJ-418 in C3H mice after subcutaneous administration was determined to be 160 mg/kg, showing that this analog was well tolerated and can be further evaluated to assess its potential therapeutic effect in tumor-bearing mice.
Keywords: DNA damage; apoptosis; cytotoxic activity; maximum tolerated dose; quinolinediones.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Rao K.V., Cullen W.P. Streptonigrin, an antitumor substance. I. Isolation and characterization. Antibiot. Annu. 1959;7:950–953. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

