Therapeutic Potential of Albumin Nanoparticles Encapsulated Visnagin in MDA-MB-468 Triple-Negative Breast Cancer Cells
- PMID: 37049991
- PMCID: PMC10096807
- DOI: 10.3390/molecules28073228
Therapeutic Potential of Albumin Nanoparticles Encapsulated Visnagin in MDA-MB-468 Triple-Negative Breast Cancer Cells
Abstract
Breast cancer is among the most recurrent malignancies, and its prevalence is rising. With only a few treatment options available, there is an immediate need to search for better alternatives. In this regard, nanotechnology has been applied to develop potential chemotherapeutic techniques, particularly for cancer therapy. Specifically, albumin-based nanoparticles are a developing platform for the administration of diverse chemotherapy drugs owing to their biocompatibility and non-toxicity. Visnagin, a naturally derived furanochromone, treats cancers, epilepsy, angina, coughs, and inflammatory illnesses. In the current study, the synthesis and characterization of albumin visnagin (AV) nanoparticles (NPs) using a variety of techniques such as transmission electron microscopy, UV-visible, Fourier transform infrared, energy dispersive X-ray composition analysis, field emission scanning electron microscopy, photoluminescence, X-Ray diffraction, and dynamic light scattering analyses have been carried out. The MTT test, dual AO/EB, DCFH-DA, Annexin-V-FITC/PI, Propidium iodide staining techniques as well as analysis of apoptotic proteins, antioxidant enzymes, and PI3K/Akt/mTOR signaling analysis was performed to examine the NPs' efficacy to suppress MDA-MB-468 cell lines. The NPs decreased cell viability increased the amount of ROS in the cells, disrupted membrane integrity, decreased the level of antioxidant enzymes, induced cell cycle arrest, and activated the PI3K/Akt/mTOR signaling cascade, ultimately leading to cell death. Thus, AV NPs possesses huge potential to be employed as a strong anticancer therapy alternative.
Keywords: MDA-MB-468 cell line; albumin visnagin nanoparticles; apoptosis; breast cancer; nanotechnology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






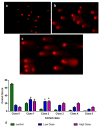



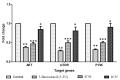
References
-
- Silvestri M., Cristaudo A., Morrone A., Messina C., Bennardo L., Nisticò S.P., Mariano M., Cameli N. Emerging skin toxicities in patients with breast cancer treated with new cyclin-dependent kinase 4/6 inhibitors: A systematic review. Drug Saf. 2021;44:725–732. doi: 10.1007/s40264-021-01071-1. - DOI - PubMed
-
- Indira K., Mudali U.K., Nishimura T., Rajendran N. A review on TiO2 nanotubes: Influence of anodization parameters, formation mechanism, properties, corrosion behavior, and biomedical applications. J. Bio-Tribo-Corros. 2015;1:28. doi: 10.1007/s40735-015-0024-x. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

