Evaluating the Application Potential of a Recombinant Ganoderma Protein as Bioactive Ingredients in Cosmetics
- PMID: 37050035
- PMCID: PMC10096787
- DOI: 10.3390/molecules28073272
Evaluating the Application Potential of a Recombinant Ganoderma Protein as Bioactive Ingredients in Cosmetics
Abstract
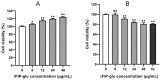
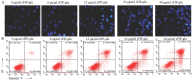
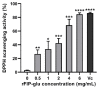
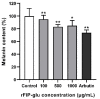
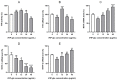
The aim of this study was to evaluate the application potential of a recombinant fungal immunomodulatory protein from Ganoderma lucidum (rFIP-glu). First, a recombinant plasmid pPIC9K::FIP-glu-His was transferred into Pichia pastoris for the production of protein. The protein was then to assess its free radical scavenging abilities and the effect on the viability of both human immortalized keratinocytes (HaCaT cells) and mouse B16-F10 melanoma cells (B16 cells) in vitro, followed by the effect on the melanin synthesis of B16 cells. The results of SDS-PAGE and western blot showed that rFIP-glu was successfully expressed. Furtherly, a bioactivity assay in vitro indicated that the scavenging rate of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals reached 84.5% at 6.0 mg/mL (p ≤ 0.0001) of rFIP-glu, showing strong antioxidant activity. Subsequently, a safety evaluation demonstrated that rFIP-glu promoted the proliferation of HaCaT cells, with the cell viability reaching 124.3% at 48 μg/mL (p ≤ 0.01), regarding the cell viability of B16 cells after exposure to rFIP-glu (48 μg/mL) significantly inhibited, to 80.7% (p ≤ 0.01). Besides, rFIP-glu inhibited the melanin synthesis of B16 cells in a dose-dependent manner from 100-1000 μg/mL, and rFIP-glu at 500 μg/mL (p ≤ 0.01) exhibited the highest intracellular melanin amount reduction of 16.8%. Furthermore, a mechanism analysis showed that rFIP-glu inhibited tyrosinase (TYR) activity by up-regulating the expression of the microphthalmia-associated transcription factor (MITF) and down-regulating the gene expression of TYR and tyrosinase-related protein-1 (TYRP-1), thus inhibiting melanin synthesis. The data implied that rFIP-glu had significant antioxidant activity and whitening potency. It should be used as raw materials for cosmeceutical applications.
Keywords: antioxidant activity; melanin; recombinant FIP-glu (rFIP-glu); tyrosinase; whitening potency.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Characterization and Functional Analysis of a Novel Fungal Immunomodulatory Protein Gene from Ganoderma leucocontextum in B16-F10 Mouse Melanoma Cells.Int J Mol Sci. 2025 May 24;26(11):5063. doi: 10.3390/ijms26115063. Int J Mol Sci. 2025. PMID: 40507874 Free PMC article.
-
Screening of engineered Pichia pastoris mutant with enhanced production of a functional rFIP-glu protein and investigating its potential bioactivities.Lett Appl Microbiol. 2021 Dec;73(6):770-778. doi: 10.1111/lam.13572. Epub 2021 Oct 10. Lett Appl Microbiol. 2021. PMID: 34597432
-
Recombinant Expression and Bioactivity Comparison of Four Typical Fungal Immunomodulatory Proteins from Three Main Ganoderma Species.BMC Biotechnol. 2018 Dec 14;18(1):80. doi: 10.1186/s12896-018-0488-0. BMC Biotechnol. 2018. PMID: 30547780 Free PMC article.
-
Molecular cloning, codon-optimized gene expression, and bioactivity assessment of two novel fungal immunomodulatory proteins from Ganoderma applanatum in Pichia.Appl Microbiol Biotechnol. 2018 Jul;102(13):5483-5494. doi: 10.1007/s00253-018-9022-5. Epub 2018 Apr 28. Appl Microbiol Biotechnol. 2018. PMID: 29705959
-
Optimization of the fermentation parameters for the production of Ganoderma lucidum immunomodulatory protein by Pichia pastoris.Prep Biochem Biotechnol. 2020;50(4):357-364. doi: 10.1080/10826068.2019.1703194. Epub 2019 Dec 17. Prep Biochem Biotechnol. 2020. PMID: 31846385
Cited by
-
Characterization and Functional Analysis of a Novel Fungal Immunomodulatory Protein Gene from Ganoderma leucocontextum in B16-F10 Mouse Melanoma Cells.Int J Mol Sci. 2025 May 24;26(11):5063. doi: 10.3390/ijms26115063. Int J Mol Sci. 2025. PMID: 40507874 Free PMC article.
-
Mid-Infrared Spectroscopic Study of Cultivating Medicinal Fungi Ganoderma: Composition, Development, and Strain Variability of Basidiocarps.J Fungi (Basel). 2023 Dec 28;10(1):23. doi: 10.3390/jof10010023. J Fungi (Basel). 2023. PMID: 38248933 Free PMC article.
References
-
- Arung E.T., Furuta S., Ishikawa H., Tanaka H., Shimizu K. Melanin biosynthesis inhibitory and antioxidant activities of quercetin-3’-O-β-D-glucoside isolated from Allium cepa. Z. Für Nat. C. 2011;66:209–214. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

