Tuning Photophysical Properties via Positional Isomerization of the Pyridine Ring in Donor-Acceptor-Structured Aggregation-Induced Emission Luminogens Based on Phenylmethylene Pyridineacetonitrile Derivatives
- PMID: 37050045
- PMCID: PMC10096500
- DOI: 10.3390/molecules28073282
Tuning Photophysical Properties via Positional Isomerization of the Pyridine Ring in Donor-Acceptor-Structured Aggregation-Induced Emission Luminogens Based on Phenylmethylene Pyridineacetonitrile Derivatives
Abstract
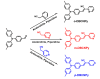
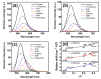
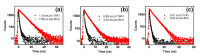
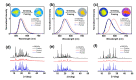
A series of aggregation-induced emission (AIE)-featured phenylmethylene pyridineacetonitrile derivatives named o-DBCNPy ((Z)-3-(4-(di-p-tolylamino)phenyl)-2-(pyridin-2-yl)acrylonitrile), m-DBCNPy ((Z)-3-(4-(di-p-tolylamino)phenyl)-2-(pyridin-3-yl)acrylonitrile), and p-DBCNPy ((Z)-3-(4-(di-p-tolylamino)phenyl)-2-(pyridin-4-yl)acrylonitrile) have been synthesized by tuning the substitution position of the pyridine ring. The linkage manner of the pyridine ring had influences on the molecular configuration and conjugation, thus leading to different photophysical properties. The absorption and fluorescence emission peak showed a bathochromic shift when the linking position of the pyridine ring changed from the meta to the ortho and para position. Meanwhile, o-DBCNPy exhibited the highest fluorescence quantum yield of 0.81 and the longest fluorescence lifetime of 7.96 ns as a neat film among all three isomers. Moreover, non-doped organic light-emitting diodes (OLEDs) were assembled in which the molecules acted as the light-emitting layer. Due to the relatively prominent emission properties, the electroluminescence (EL) performance of the o-DBCNPy-based OLED was superior to those of the devices based on the other two isomers with an external quantum efficiency (EQE) of 4.31%. The results indicate that delicate molecular modulation of AIE molecules could endow them with improved photophysical properties, making them potential candidates for organic photoelectronic devices.
Keywords: aggregation-induced emission; fluorescence emission; non-doped organic light-emitting diode; phenylmethylene pyridineacetonitrile; positional isomerization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Synthesis, photoluminescence and electroluminescence properties of a new blue emitter with aggregation-induced emission and thermally activated delayed fluorescence characteristics.Spectrochim Acta A Mol Biomol Spectrosc. 2023 Apr 15;291:122344. doi: 10.1016/j.saa.2023.122344. Epub 2023 Jan 11. Spectrochim Acta A Mol Biomol Spectrosc. 2023. PMID: 36682256
-
Unconventional Three-Armed Luminogens Exhibiting Both Aggregation-Induced Emission and Thermally Activated Delayed Fluorescence Resulting in High-Performing Solution-Processed Organic Light-Emitting Diodes.ACS Appl Mater Interfaces. 2018 May 2;10(17):14966-14977. doi: 10.1021/acsami.7b19681. Epub 2018 Apr 23. ACS Appl Mater Interfaces. 2018. PMID: 29630336
-
[1,2,4]Triazolo[1,5- a]pyridine-Based Host Materials for Green Phosphorescent and Delayed-Fluorescence OLEDs with Low Efficiency Roll-Off.ACS Appl Mater Interfaces. 2018 Jul 25;10(29):24689-24698. doi: 10.1021/acsami.8b07462. Epub 2018 Jul 13. ACS Appl Mater Interfaces. 2018. PMID: 29974742
-
Red/Near-Infrared Thermally Activated Delayed Fluorescence OLEDs with Near 100 % Internal Quantum Efficiency.Angew Chem Int Ed Engl. 2019 Oct 7;58(41):14660-14665. doi: 10.1002/anie.201906575. Epub 2019 Aug 5. Angew Chem Int Ed Engl. 2019. PMID: 31313424 Review.
-
Recent Advances in the Z/E Isomers of Tetraphenylethene Derivatives: Stereoselective Synthesis, AIE Mechanism, Photophysical Properties, and Application as Chemical Probes.Chem Asian J. 2019 Aug 1;14(15):2524-2541. doi: 10.1002/asia.201900282. Epub 2019 Jun 26. Chem Asian J. 2019. PMID: 30994248 Review.
Cited by
-
Synthesis and Characterization of Symmetrical N-Heterocyclic Carbene Copper(II) Complexes-An Investigation of the Influence of Pyridinyl Substituents.Molecules. 2024 Jul 27;29(15):3542. doi: 10.3390/molecules29153542. Molecules. 2024. PMID: 39124947 Free PMC article.
-
The Application of Hydrogen Sulfide Fluorescent Probe in Food Preservation, Detection and Evaluation.Molecules. 2024 Aug 22;29(16):3973. doi: 10.3390/molecules29163973. Molecules. 2024. PMID: 39203051 Free PMC article. Review.
-
Polymers Containing Phenothiazine, Either as a Dopant or as Part of Their Structure, for Dye-Sensitized and Bulk Heterojunction Solar Cells.Polymers (Basel). 2024 Aug 15;16(16):2309. doi: 10.3390/polym16162309. Polymers (Basel). 2024. PMID: 39204529 Free PMC article. Review.
-
Designing Thiadiazoloquinoxaline-Based Conjugated Polymers for Efficient Organic Photovoltaics: A DFT/TDDFT Study.Molecules. 2024 Apr 1;29(7):1580. doi: 10.3390/molecules29071580. Molecules. 2024. PMID: 38611860 Free PMC article.
-
Turn-On Fluorescence Probe for Cancer-Related γ-Glutamyltranspeptidase Detection.Molecules. 2024 Oct 9;29(19):4776. doi: 10.3390/molecules29194776. Molecules. 2024. PMID: 39407704 Free PMC article.
References
-
- Fei N., Wei Q., Cao L., Bai Y., Ji H.L., Peng R.X., Huang L., Hao S.Y., Ge Z.Y. A symmetric nonpolar blue AIEgen as nondoped fluorescent OLED emitter with low efficiency roll-off. Org. Electron. 2020;78:105574. doi: 10.1016/j.orgel.2019.105574. - DOI
-
- Liu R.R., Jing J.B., Zhang S., Wang K., Tian W.J., Yang P. Aggregation-induced emission of a 2D protein supramolecular nanofilm with emergent functions. Mater. Chem. Front. 2020;4:1256. doi: 10.1039/D0QM00031K. - DOI
Grants and funding
- 22105171/National Natural Science Foundation of China
- LQ20B020002/Natural Science Foundation of Zhejiang Province
- LQ19E030001/Natural Science Foundation of Zhejiang Province
- 2022C01177/Key R&D Project for Tackling Key Scientific and Technological Problems of Zhejiang Province, the "Leading Swan Goose Project"
- CY2022355/Zhejiang Administration for Market Regulation Eyas Program Cultivation Project
LinkOut - more resources
Full Text Sources
Miscellaneous

