The Role of Mucoadhesion and Mucopenetration in the Immune Response Induced by Polymer-Based Mucosal Adjuvants
- PMID: 37050229
- PMCID: PMC10097111
- DOI: 10.3390/polym15071615
The Role of Mucoadhesion and Mucopenetration in the Immune Response Induced by Polymer-Based Mucosal Adjuvants
Abstract
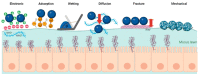
Mucus is a viscoelastic gel that acts as a protective barrier for epithelial surfaces. The mucosal vehicles and adjuvants need to pass through the mucus layer to make drugs and vaccine delivery by mucosal routes possible. The mucoadhesion of polymer particle adjuvants significantly increases the contact time between vaccine formulations and the mucosa; then, the particles can penetrate the mucus layer and epithelium to reach mucosa-associated lymphoid tissues. This review presents the key findings that have aided in understanding mucoadhesion and mucopenetration while exploring the influence of physicochemical characteristics on mucus-polymer interactions. We describe polymer-based particles designed with mucoadhesive or mucopenetrating properties and discuss the impact of mucoadhesive polymers on local and systemic immune responses after mucosal immunization. In future research, more attention paid to the design and development of mucosal adjuvants could lead to more effective vaccines.
Keywords: immune response; mucoadhesion; mucosal adjuvants; mucosal vaccines; polymeric particles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Untangling Mucosal Drug Delivery: Engineering, Designing, and Testing Nanoparticles to Overcome the Mucus Barrier.ACS Biomater Sci Eng. 2022 Apr 11;8(4):1396-1426. doi: 10.1021/acsbiomaterials.2c00047. Epub 2022 Mar 16. ACS Biomater Sci Eng. 2022. PMID: 35294187 Review.
-
Mucoadhesive vs. mucopenetrating particulate drug delivery.Eur J Pharm Biopharm. 2016 Jan;98:76-89. doi: 10.1016/j.ejpb.2015.11.003. Epub 2015 Nov 17. Eur J Pharm Biopharm. 2016. PMID: 26598207 Review.
-
Mucoadhesive-to-Mucopenetrating Nanoparticles for Mucosal Drug Delivery: A Mini Review.Int J Nanomedicine. 2025 Feb 20;20:2241-2252. doi: 10.2147/IJN.S505427. eCollection 2025. Int J Nanomedicine. 2025. PMID: 39995958 Free PMC article. Review.
-
Mucoadhesive versus mucopenetrating nanoparticles for oral delivery of insulin.Acta Biomater. 2021 Nov;135:506-519. doi: 10.1016/j.actbio.2021.08.046. Epub 2021 Sep 4. Acta Biomater. 2021. PMID: 34487859
-
A Polymer Chemistry Point of View on Mucoadhesion and Mucopenetration.Macromol Biosci. 2017 Sep;17(9). doi: 10.1002/mabi.201700060. Epub 2017 Jul 4. Macromol Biosci. 2017. PMID: 28675773 Review.
Cited by
-
Optimization of Polylactide-Co-Glycolide-Rifampicin Nanoparticle Synthesis, In Vitro Study of Mucoadhesion and Drug Release.Polymers (Basel). 2024 Aug 30;16(17):2466. doi: 10.3390/polym16172466. Polymers (Basel). 2024. PMID: 39274099 Free PMC article.
-
In Situ Gelling Dexamethasone Oromucosal Formulation: Physical Characteristics Influencing Drug Delivery.Gels. 2025 Jan 2;11(1):26. doi: 10.3390/gels11010026. Gels. 2025. PMID: 39851997 Free PMC article.
-
Muco-Adhesive and Muco-Penetrative Formulations for the Oral Delivery of Insulin.ACS Omega. 2024 May 29;9(23):24121-24141. doi: 10.1021/acsomega.3c10305. eCollection 2024 Jun 11. ACS Omega. 2024. PMID: 38882129 Free PMC article. Review.
-
The Use of Particulate Systems for Tuberculosis Prophylaxis and Treatment: Opportunities and Challenges.Microorganisms. 2023 Aug 2;11(8):1988. doi: 10.3390/microorganisms11081988. Microorganisms. 2023. PMID: 37630548 Free PMC article. Review.
-
Multilayer Adjuvanted Influenza Protein Nanoparticles Improve Intranasal Delivery and Antigen-Specific Immunity.ACS Nano. 2025 Feb 25;19(7):7005-7025. doi: 10.1021/acsnano.4c14735. Epub 2025 Feb 15. ACS Nano. 2025. PMID: 39954231 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

