Strategy Based on Michael Addition Reaction for the Development of Bioinspired Multilayered and Multiphasic 3D Constructs
- PMID: 37050249
- PMCID: PMC10096539
- DOI: 10.3390/polym15071635
Strategy Based on Michael Addition Reaction for the Development of Bioinspired Multilayered and Multiphasic 3D Constructs
Abstract
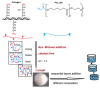
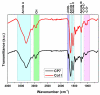
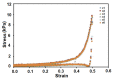
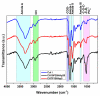
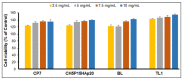
The high incidence of osteochondral defects has increased the interest in the development of improved repairing alternatives, with tissue engineering being considered a promising approach. The hierarchical, complex structure of osteochondral tissue requires the design of a biomimetic multilayered scaffold. Here, a multilayered and multiphasic 3D macroporous structure was achieved at subzero temperature by the Michael addition reaction of amino functionalities of collagen with acryloyl groups of a bifunctionalized poly(ε-caprolactone). This green approach has been successfully applied to crosslink layers of different composition, both for their efficient sequential formation and connection. Polyethylenimine functionalized nano-hydroxyapatite (nHApLPEI) was added to the bottom layer. The resulting hybrid cryogels were characterized by morphology, equilibrium swelling ratios, compressive strength analysis, and MTS assay. They presented good stability, integrity, and biocompatibility. The results revealed that the properties of the prepared constructs may be tuned by varying the composition, number, and thickness of the layers. The Young modulus values were between 3.5 ± 0.02 and 10.5 ± 0.6 kPa for the component layers, while for the multilayered structures they were more than 7.3 ± 0.2 kPa. The equilibrium swelling ratio varied between 4.6 and 14.2, with a value of ~10.5 for the trilayered structure, correlated with the mean pore sizes (74-230 µm).
Keywords: aza-Michael addition; biocomposite; cryogelation; multilayered construct; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
[Preparation and in vitro evaluation of tissue engineered osteochondral integration of multi-layered scaffold].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018 Apr 15;32(4):434-440. doi: 10.7507/1002-1892.201712038. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018. PMID: 29806301 Free PMC article. Chinese.
-
Integrated trilayered silk fibroin scaffold for osteochondral differentiation of adipose-derived stem cells.ACS Appl Mater Interfaces. 2014 Oct 8;6(19):16696-705. doi: 10.1021/am5036708. Epub 2014 Sep 25. ACS Appl Mater Interfaces. 2014. PMID: 25210952
-
Three-dimensional printed multiphasic scaffolds with stratified cell-laden gelatin methacrylate hydrogels for biomimetic tendon-to-bone interface engineering.J Orthop Translat. 2020 Feb 8;23:89-100. doi: 10.1016/j.jot.2020.01.004. eCollection 2020 Jul. J Orthop Translat. 2020. PMID: 32514393 Free PMC article.
-
Current strategies in multiphasic scaffold design for osteochondral tissue engineering: A review.J Biomed Mater Res A. 2015 Jul;103(7):2460-81. doi: 10.1002/jbm.a.35356. Epub 2014 Nov 11. J Biomed Mater Res A. 2015. PMID: 25345589 Review.
-
Advances and prospects in biomimetic multilayered scaffolds for articular cartilage regeneration.Regen Biomater. 2020 Sep 30;7(6):527-542. doi: 10.1093/rb/rbaa042. eCollection 2020 Dec. Regen Biomater. 2020. PMID: 33365139 Free PMC article. Review.
Cited by
-
Next-Generation Wound Healing Materials: Role of Biopolymers and Their Composites.Polymers (Basel). 2025 Aug 19;17(16):2244. doi: 10.3390/polym17162244. Polymers (Basel). 2025. PMID: 40871190 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

