Determination of Hyaluronic Acid Dermal Filler Impurities Using SEM/EDS Analysis
- PMID: 37050263
- PMCID: PMC10096933
- DOI: 10.3390/polym15071649
Determination of Hyaluronic Acid Dermal Filler Impurities Using SEM/EDS Analysis
Abstract
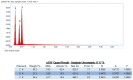
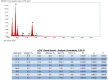
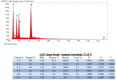
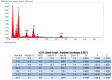
Although hyaluronic acid (HA) filler injections are associated with various non-vascular inflammatory complications, the underlying mode of action remains unclear. The hyaluronic acid filler may not be sufficiently pure, leading to an immune response. The present study attempted to identify any impurities in hyaluronic acid fillers available on the market. Particles were counted after degrading hyaluronic acid filler with hyaluronidase. Particulate matter was subsequently observed using scanning electron microscopy, and the particle components were evaluated using energy-dispersive X-ray spectroscopy. Different quantities of impurity particles (>10 and 25 μm) were detected microscopically. Silicon and aluminum isotopes were also detected. Hyaluronic acid fillers were contaminated with these particles. The degree of contamination varied substantially among the tested filler products. These contaminant particles may evoke reactions in the patient's body. Clinicians should be aware of this source of possible contamination and its effects.
Keywords: filler complications; hyaluronic acid filler; impurities; scanning electron microscopy.
Conflict of interest statement
The authors have the following to disclose: Won Lee has been an investigator, speaker, and consultant for Joonghun Pharmaceutical, Seoul, South Korea. The L’ Orient hyaluronic acid filler was provided by Joonghun Pharmaceutical, Seoul, South Korea. Other hyaluronic acid fillers were also purchased.
Figures









References
-
- Koh I.S., Lee W. Filler Complications: Filler-Induced Hypersensitivity Reactions, Granuloma, Necrosis, and Blindness. 3rd ed. Springer; Berlin, Germany: 2019.
Grants and funding
LinkOut - more resources
Full Text Sources

