Supramolecular Linear-Dendritic Nanoreactors: Synthesis and Catalytic Activity in "Green" Suzuki-Miyaura Reactions
- PMID: 37050285
- PMCID: PMC10096851
- DOI: 10.3390/polym15071671
Supramolecular Linear-Dendritic Nanoreactors: Synthesis and Catalytic Activity in "Green" Suzuki-Miyaura Reactions
Abstract
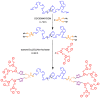
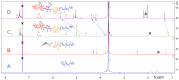
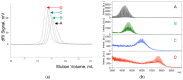
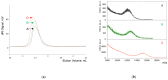
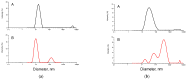
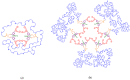
This study describes the synthesis of novel amphiphilic linear-dendritic block copolymers and their self-assembly in water to form supramolecular nanoreactors capable of catalyzing Suzuki-Miyaura coupling reactions under "green" conditions. The block copolymers were formed through copper(I)-catalyzed alkyne-azide cycloaddition between azide functionalized poly(benzyl ether) dendrons as the perfectly branched blocks, as well as bis-alkyne modified poly(ethylene glycol), PEG, as the linear block. A first-generation poly(benzyl ether) dendron (G1) was coupled to a bis-alkyne modified PEG with molecular mass of 5 kDa, forming an ABA copolymer (G1)2-PEG5k-(G1)2 (yield 62%), while a second-generation dendron (G2) was coupled to a 11 kDa bis-alkyne modified PEG to produce (G2)2-PEG11k-(G2)2 (yield 49%). The structural purity and low dispersity of the linear-dendritic copolymers were verified by size-exclusion chromatography and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Their self-assembly was studied by dynamic light scattering, showing that (G1)2-PEG5k-(G1)2 and (G2)2-PEG11k-(G2)2 formed single populations of micelles (17 nm and 37 nm in diameter, respectively). The triazole rings located at the boundaries between the core and the corona are efficient chelating groups for transition metals. The ability of the micelles to complex Pd was confirmed by 1H NMR, transmission electron microscopy, and inductively coupled plasma. The catalytic activity of the supramolecular linear-dendritic/Pd complexes was tested in water by model Suzuki-Miyaura reactions in which quantitative yields were achieved within 3 h at 40 °C, while, at 17 °C, a yield of more than 70% was attained after 17 h.
Keywords: Suzuki coupling; block copolymer; linear-dendritic; self-assembly; “green” chemistry.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
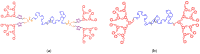


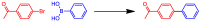
References
-
- Gitsov I., Fréchet J.M.J. Solution and solid-state properties of hybrid linear-dendritic block copolymers. Macromolecules. 1993;26:6536–6546. doi: 10.1021/ma00076a035. - DOI
-
- van Hest J.C.M., Baars M.W.P.L., Elissen-Roman C., van Genderen M.H.P., Meijer E.W. Acid-Functionalized Amphiphiles, Derived from Polystyrene-Poly(propylene imine) Dendrimers, with a pH-Dependent Aggregation. Macromolecules. 1995;28:6689–6691. doi: 10.1021/ma00123a043. - DOI
-
- Gitsov I. Synthesis and Self-Assembly of Linear-Dendritic Hybrid Polymers. In: Kobayashi S., Müllen K., editors. Encyclopedia of Polymeric Nanomaterials. Springer; Berlin/Heidelberg, Germany: 2015. pp. 2436–2446. - DOI
-
- Liu X., Gitsov I. Nonionic Amphiphilic Linear-Dendritic Block Copolymers. Solvent-Induced Self-Assembly and Morphology Tuning. Macromolecules. 2019;52:5563–5573. doi: 10.1021/acs.macromol.9b01023. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

