Nucleus-Targeting Nanoplatform Based on Dendritic Peptide for Precise Photothermal Therapy
- PMID: 37050365
- PMCID: PMC10096676
- DOI: 10.3390/polym15071753
Nucleus-Targeting Nanoplatform Based on Dendritic Peptide for Precise Photothermal Therapy
Abstract
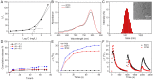
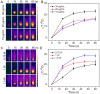
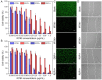
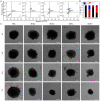
Photothermal therapy directly acting on the nucleus is a potential anti-tumor treatment with higher killing efficiency. However, in practical applications, it is often difficult to achieve precise nuclear photothermal therapy because agents are difficult to accurately anchor to the nucleus. Therefore, it is urgent to develop a nanoheater that can accurately locate the nucleus. Here, we designed an amphiphilic arginine-rich dendritic peptide (RDP) with the sequence CRRK(RRCG(Fmoc))2, and prepared a nucleus-targeting nanoplatform RDP/I by encapsulating the photothermal agent IR780 in RDP for precise photothermal therapy of the tumor nucleus. The hydrophobic group Fmoc of the dendritic peptide provides strong hydrophobic force to firmly encapsulate IR780, which improves the solubility and stability of IR780. Moreover, the arginine-rich structure facilitates cellular uptake of RDP/I and endows it with the ability to quickly anchor to the nucleus. The nucleus-targeting nanoplatform RDP/I showed efficient nuclear enrichment ability and a significant tumor inhibition effect.
Keywords: arginine-rich dendritic peptide; nuclear localization; precise photothermal therapy; tumor inhibition.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Jing R.W., Wang Q., Chen L., Li G.T., Li R.B., Zhang L.J., Zhang H.B., Zuo B.F., Seow Y.Q., Qiao X., et al. Functional imaging and targeted drug delivery in mice and patient tumors with a cell nucleolus-localizing and tumor-targeting peptide. Biomaterials. 2022;289:121758. doi: 10.1016/j.biomaterials.2022.121758. - DOI - PubMed
-
- Du W., Du S.B., Dong X., Bai H., Jiang J.M., Hao S.P., Yang F., Xiao Q.C., Zhang B., Ge J.Y., et al. Biodegradable silica nanocapsules enable efficient nuclear-targeted delivery of native proteins for cancer therapy. Biomaterials. 2023;294:122000. doi: 10.1016/j.biomaterials.2023.122000. - DOI - PubMed
-
- Zhang Z.J., Xu W.H., Xiao P.H., Kang M.M., Yan D.Y., Wen H.F., Song N., Wang D., Tang B.Z. Molecular engineering of high-performance aggregation-induced emission photosensitizers to boost cancer theranostics mediated by acid-triggered nucleus-targeted nanovectors. ACS Nano. 2021;15:10689–10699. doi: 10.1021/acsnano.1c03700. - DOI - PubMed
-
- Gao F., Yang X., Luo X.P., Xue X.L., Qian C.G., Sun M.J. Photoactivated nanosheets accelerate nucleus access of cisplatin for drug-resistant cancer therapy. Adv. Funct. Mater. 2020;30:2001546. doi: 10.1002/adfm.202001546. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

