Sensing Mechanisms of Rough Plasmonic Surfaces for Protein Binding of Surface Plasmon Resonance Detection
- PMID: 37050437
- PMCID: PMC10098771
- DOI: 10.3390/s23073377
Sensing Mechanisms of Rough Plasmonic Surfaces for Protein Binding of Surface Plasmon Resonance Detection
Abstract
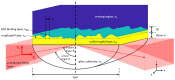
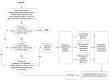
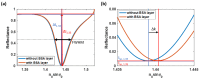
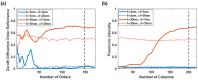
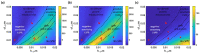
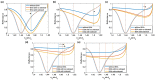
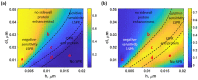
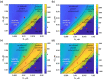
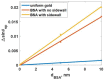
Surface plasmon resonance (SPR) has been utilized in various optical applications, including biosensors. The SPR-based sensor is a gold standard for protein kinetic measurement due to its ultrasensitivity on the plasmonic metal surface. However, a slight change in the surface morphology, such as roughness or pattern, can significantly impact its performance. This study proposes a theoretical framework to explain sensing mechanisms and quantify sensing performance parameters of angular surface plasmon resonance detection for binding kinetic sensing at different levels of surface roughness. The theoretical investigation utilized two models, a protein layer coating on a rough plasmonic surface with and without sidewall coatings. The two models enable us to separate and quantify the enhancement factors due to the localized surface plasmon polaritons at sharp edges of the rough surfaces and the increased surface area for protein binding due to roughness. The Gaussian random surface technique was employed to create rough metal surfaces. Reflectance spectra and quantitative performance parameters were simulated and quantified using rigorous coupled-wave analysis and Monte Carlo simulation. These parameters include sensitivity, plasmonic dip position, intensity contrast, full width at half maximum, plasmonic angle, and figure of merit. Roughness can significantly impact the intensity measurement of binding kinetics, positively or negatively, depending on the roughness levels. Due to the increased scattering loss, a tradeoff between sensitivity and increased roughness leads to a widened plasmonic reflectance dip. Some roughness profiles can give a negative and enhanced sensitivity without broadening the SPR spectra. We also discuss how the improved sensitivity of rough surfaces is predominantly due to the localized surface wave, not the increased density of the binding domain.
Keywords: binding-kinetics sensitivity; quantitative-sensing performance; sensing mechanisms; sensitivity-enhancement mechanisms; surface plasmon resonance; surface roughness.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Brongersma M.L., Kik P.G. Surface Plasmon Nanophotonics. Volume 131 Springer; Dordrecht, The Netherlands: 2007.
-
- Somekh M.G., Pechprasarn S. Handbook of Photonics for Biomedical Engineering. Springer; Dordrecht, The Netherlands: 2017. Surface plasmon, surface wave, and enhanced evanescent wave microscopy; pp. 503–543.
-
- Homola J., Yee S.S., Gauglitz G. Surface plasmon resonance sensors. Sens. Actuators B Chem. 1999;54:3–15. doi: 10.1016/S0925-4005(98)00321-9. - DOI
-
- Suda Y., Arano A., Fukui Y., Koshida S., Wakao M., Nishimura T., Kusumoto S., Sobel M. Immobilization and clustering of structurally defined oligosaccharides for sugar chips: An improved method for surface plasmon resonance analysis of protein-carbohydrate interactions. Bioconjug. Chem. 2006;17:1125–1135. doi: 10.1021/bc0600620. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

