Bacterial Colony Phenotyping with Hyperspectral Elastic Light Scattering Patterns
- PMID: 37050545
- PMCID: PMC10098818
- DOI: 10.3390/s23073485
Bacterial Colony Phenotyping with Hyperspectral Elastic Light Scattering Patterns
Abstract
The elastic light-scatter (ELS) technique, which detects and discriminates microbial organisms based on the light-scatter pattern of their colonies, has demonstrated excellent classification accuracy in pathogen screening tasks. The implementation of the multispectral approach has brought further advantages and motivated the design and validation of a hyperspectral elastic light-scatter phenotyping instrument (HESPI). The newly developed instrument consists of a supercontinuum (SC) laser and an acousto-optic tunable filter (AOTF). The use of these two components provided a broad spectrum of excitation light and a rapid selection of the wavelength of interest, which enables the collection of multiple spectral patterns for each colony instead of relying on single band analysis. The performance was validated by classifying microflora of green-leafed vegetables using the hyperspectral ELS patterns of the bacterial colonies. The accuracy ranged from 88.7% to 93.2% when the classification was performed with the scattering pattern created at a wavelength within the 473-709 nm region. When all of the hyperspectral ELS patterns were used, owing to the vastly increased size of the data, feature reduction and selection algorithms were utilized to enhance the robustness and ultimately lessen the complexity of the data collection. A new classification model with the feature reduction process improved the overall classification rate to 95.9%.
Keywords: bacterial colony phenotyping; bacterial identification; elastic light scattering; hyperspectral imaging; light diffraction; optical sensing; supercontinuum laser.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




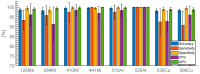




References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

