The Efficacy and Safety of Imusil® Tablets in the Treatment of Adult Patients With Mild COVID-19: A Prospective, Randomized, Multicenter, Open-Label Study
- PMID: 37051002
- PMCID: PMC10085312
- DOI: 10.7759/cureus.35881
The Efficacy and Safety of Imusil® Tablets in the Treatment of Adult Patients With Mild COVID-19: A Prospective, Randomized, Multicenter, Open-Label Study
Abstract
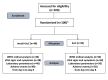
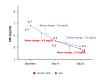
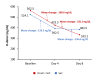
Introduction Coronavirus disease 2019 (COVID-19) is a serious concern of the new era. Along with antiviral synthetic medications, there is a need to discover efficacious herbal antiviral medicines with minimum side effects in patients against COVID-19. This study aimed to assess the efficacy and safety of Imusil® among patients with mild COVID-19. Methods A prospective, randomized, multicenter, open-label, interventional study was conducted in patients with mild COVID-19 infection. Patients received either Imusil one tablet four times a day (seven days) along with the standard of care (SoC) or only SoC. The study endpoints were reverse transcription-polymerase chain reaction (RT-PCR) negativity, changes in cycle threshold (CT), clinical improvement, change in blood inflammatory indexes, and safety assessment. Results A total of 100 patients were enrolled, and 98 received at least one dose of treatment. The median age of patients was 36.0 years, and 58 were males. By day 4, 85.4% of patients in the Imusil+SoC group tested negative for RT-PCR compared to 64% of patients exhibiting the same outcome in the SoC group (P=0.0156). After eight days, clinical improvement was observed in all patients from the Imusil+SoC group, while in the SoC group, clinical improvement was observed in 94.0% of patients (P=0.4947). During follow-up visits, the average C-reactive protein (CRP) levels decreased from baseline in both treatment groups. The decrease in the levels of CRP (-7.3 mg/dL versus -5.5 mg/dL), D-dimer (-231.0 ng/mL versus -151.6 ng/mL), and interleukin 6 (IL-6) (-2.3 pg/mL versus -2.0 pg/mL) at eight days was comparatively higher in the Imusil+SoC group versus the SoC group. There were no serious treatment-emergent adverse events in the drug arm. Conclusion Imusil provides effective antiviral activity and safety in mild COVID-19 patients. Imusil ensures faster RT-PCR negativity and clinical improvement and ensures effective reduction of inflammatory markers such as CRP, D-dimer and interleukin 6.
Keywords: crp; d-dimer; interleukin 6; rt-pcr; safe.
Copyright © 2023, Abhyankar et al.
Conflict of interest statement
The authors have declared financial relationships, which are detailed in the next section.
Figures

References
-
- Combating oxidative stress and inflammation in COVID-19 by molecular hydrogen therapy: mechanisms and perspectives. Alwazeer D, Liu FF, Wu XY, LeBaron TW. https://doi.org/10.1155/2021/5513868. Oxid Med Cell Longev. 2021;2021:5513868. - PMC - PubMed
-
- Real-world experience with favipiravir for the treatment of mild-to-moderate COVID-19 in India. Joshi S, Vora A, Venugopal K, et al. https://doi.org/10.2147/POR.S364066. Pragmat Obs Res. 2022;13:33–41. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
