LDH as an adjuvant makes Brucella outer-membrane vesicles and outer-membrane vesicle-associated proteins highly protective in mice
- PMID: 37051103
- PMCID: PMC10083835
- DOI: 10.22038/IJBMS.2023.67394.14775
LDH as an adjuvant makes Brucella outer-membrane vesicles and outer-membrane vesicle-associated proteins highly protective in mice
Abstract
Objectives: Existing Brucella vaccines are attenuated and can cause vaccine-associated brucellosis; and these safety concerns have affected their application. Although subunit vaccines have the advantages of safety, efficacy, low cost, and rapid production, they are usually poorly immunogenic and insufficient to trigger persistent immunity. Therefore, we added layered double hydroxide (LDH) as an adjuvant to Brucella subunit vaccine formulations to enhance the immune response to the antigen.
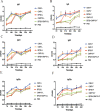
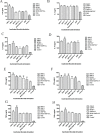
Materials and methods: LDH and Freund's adjuvant were combined with Brucella outer-membrane vesicles (OMVs) and OMV-associated proteins to form a subunit vaccine, respectively. The immunogenicity of LDH as an adjuvant was assessed in BALB/c mice. We examined levels of immunoglobulin G, G1, and G2a (IgG, IgG1, and IgG2a) antibodies (aBs); percentages of Cluster of Differentiation 4-positive (CD4+) and CD8+ T cells in peripheral-blood lymphocytes; and secretion of cytokines in mouse spleen lymphocytes. Finally, splenic index and splenic bacterial load were assessed via Brucella challenge experiments on mice.
Results: The LDH subunit vaccine also produced high levels of specific aBs in mice compared with Freund's adjuvant subunit vaccine and induced mainly T-helper 1 cell (Th1)-type immune responses. In addition, mice in the LDH subunit vaccine group had significantly lower bacterial loads in their spleens than those in the Freund's adjuvant subunit vaccine group, and the LDH-OMV vaccine offered a higher level of protection against Brucella attack.
Conclusion: LDH as an adjuvant-paired vaccine provided a high level of protection against Brucella infection.
Keywords: Adjuvant; Brucella; Immunogenicity; Outer-membrane vesicles; Vaccine.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures



Similar articles
-
Non-infectious outer membrane vesicles derived from Brucella abortus S19Δper as an alternative acellular vaccine protects mice against virulent challenge.Int Immunopharmacol. 2021 Jan;90:107148. doi: 10.1016/j.intimp.2020.107148. Epub 2020 Nov 12. Int Immunopharmacol. 2021. PMID: 33189614
-
Immunization with recombinant Brucella species outer membrane protein Omp16 or Omp19 in adjuvant induces specific CD4+ and CD8+ T cells as well as systemic and oral protection against Brucella abortus infection.Infect Immun. 2009 Jan;77(1):436-45. doi: 10.1128/IAI.01151-08. Epub 2008 Nov 3. Infect Immun. 2009. PMID: 18981242 Free PMC article.
-
Vaccination with the recombinant Brucella outer membrane protein 31 or a derived 27-amino-acid synthetic peptide elicits a CD4+ T helper 1 response that protects against Brucella melitensis infection.Infect Immun. 2005 Dec;73(12):8079-88. doi: 10.1128/IAI.73.12.8079-8088.2005. Infect Immun. 2005. PMID: 16299302 Free PMC article.
-
Recombinant outer membrane protein 25c from Brucella abortus induces Th1 and Th2 mediated protection against Brucella abortus infection in mouse model.Mol Immunol. 2018 Jul;99:9-18. doi: 10.1016/j.molimm.2018.04.002. Epub 2018 Apr 9. Mol Immunol. 2018. PMID: 29649688
-
Intraperitoneal administration of a novel chimeric immunogen (rOP) elicits IFN-γ and IL-12p70 protective immune response in BALB/c mice against virulent Brucella.Immunol Lett. 2017 Dec;192:79-87. doi: 10.1016/j.imlet.2017.10.013. Epub 2017 Oct 26. Immunol Lett. 2017. PMID: 29106986
Cited by
-
Covalent Functionalization of Layered Double Hydroxides to Generate Peptide-Based SARS-CoV-2 Nanovaccine.Materials (Basel). 2025 May 23;18(11):2449. doi: 10.3390/ma18112449. Materials (Basel). 2025. PMID: 40508447 Free PMC article.
-
Next-generation aluminum adjuvants: Immunomodulatory layered double hydroxide NanoAlum reengineered from first-line drugs.Acta Pharm Sin B. 2024 Nov;14(11):4665-4682. doi: 10.1016/j.apsb.2024.09.012. Epub 2024 Sep 14. Acta Pharm Sin B. 2024. PMID: 39664431 Free PMC article. Review.
References
-
- Seleem MN, Boyle SM, Sriranganathan N. Brucellosis: A re-emerging zoonosis. Vet Microbiol. 2010;140:392–398. - PubMed
-
- Galinska EM, Zagórski J. Brucellosis in humans-etiology, diagnostics, clinical forms. Ann Agric Environ Med. 2013;20:233–238. - PubMed
-
- Doganay M, Aygen B. Human brucellosis: An overview. Int J Infec Dis. 2003;7:173–182.
-
- Hou H, Liu X, Peng Q. The advances in brucellosis vaccines. Vaccine. 2019;37:3981–3988. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
