Long-term outcomes of liver transplantation using grafts from donors with active hepatitis B virus replication: a multicenter cohort study
- PMID: 37051154
- PMCID: PMC10083344
- DOI: 10.4174/astr.2023.104.4.183
Long-term outcomes of liver transplantation using grafts from donors with active hepatitis B virus replication: a multicenter cohort study
Abstract
Purpose: Liver grafts from donors with HBV infection contributed to expanding the donor pool under the hepatitis B immunoglobulin and antiviral agents (nucleos(t)ide analogues) in the HBV-endemic area. We report long-term outcomes of liver transplantations (LTs) using grafts from donors with active or chronic HBV infection.
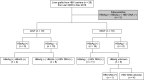
Methods: Overall, 2,260 LTs performed in 3 major hospitals in Seoul from January 2000 to April 2019 were assessed for inclusion. Twenty-six grafts (1.2%) were obtained from HBsAg (+), HBeAb (+), or HBcAb (+) donors, and recipient outcomes were retrospectively reviewed. Donor and recipient demographics and transplantation outcomes were analyzed.
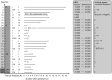
Results: Sixteen deceased donor LTs were performed using active HBsAg (+) grafts. Ten other LTs were sourced from 10 living donors. There was no significant difference in survival in patients who received deceased donor LTs compared with that in those who underwent LT with non-hepatitis virus-infected grafts. Fourteen patients who were followed up for >5 years were stable, and no difference in hepatocellular carcinoma recurrence rate was observed 5 years after transplantation between transplants from donors with and those without HBV.
Conclusion: Considering long-term outcomes, liver grafts from donors with active HBV replication can be safely used for LT.
Keywords: Hepatitis B virus; Hepatocellular carcinoma; Liver transplantation; Marginal graft; Outcome.
Copyright © 2023, the Korean Surgical Society.
Conflict of interest statement
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
Figures

References
-
- Lee WC, Chou HS, Wu TJ, Lee CS, Lee CF, Chan KM. Indicators and outcome of liver transplantation in acute liver decompensation after flares of hepatitis B. J Viral Hepat. 2011;18:193–199. - PubMed
-
- Chan KM, Yu MC, Chou HS, Wu TJ, Lee CF, Lee WC. Significance of tumor necrosis for outcome of patients with hepatocellular carcinoma receiving locoregional therapy prior to liver transplantation. Ann Surg Oncol. 2011;18:2638–2646. - PubMed
-
- British Transplantation Society (BTS) Guidelines for hepatitis B & solid organ transplantation. BTS; 2018. - PubMed
-
- Hashimoto K, Miller C. The use of marginal grafts in liver transplantation. J Hepatobiliary Pancreat Surg. 2008;15:92–101. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

