Impact of muscular symptoms and/or pain on disease characteristics, disability, and quality of life in adult patients with hypophosphatasia: A cross-sectional analysis from the Global HPP Registry
- PMID: 37051203
- PMCID: PMC10083387
- DOI: 10.3389/fendo.2023.1138599
Impact of muscular symptoms and/or pain on disease characteristics, disability, and quality of life in adult patients with hypophosphatasia: A cross-sectional analysis from the Global HPP Registry
Abstract
Introduction: Hypophosphatasia (HPP) manifests in adults as fractures/pseudofractures, pain, muscle weakness, and other functional impairments. Better phenotypic disease characterization is needed to help recognize disability and treat patients with HPP.
Methods: Baseline/pretreatment demographic, clinical characteristic, and patient-reported disability/health-related quality-of-life (HRQoL) data from adults (≥18 y) in the Global HPP Registry (NCT02306720) were stratified by presence of overt skeletal manifestations (skeletal group) versus muscular/pain manifestations without skeletal manifestations (muscular/pain group) and summarized descriptively. Disability was measured using the Health Assessment Questionnaire-Disability Index (HAQ-DI), and HRQoL using the 36-item Short Form Health Survey (SF-36v2).
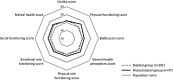
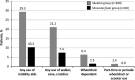
Results: Of 468 adults, 300 were classified into the skeletal group and 73 into the muscular/pain group. The skeletal group had a higher median age at baseline (50.1 vs 44.4 y; P=0.047) but a lower median age at first HPP manifestation (12.3 vs 22.1 y; P=0.0473), with more signs and symptoms (median, 4 vs 3; P<0.0001) and involved body systems (median, 3 vs 2; P<0.0001) than the muscular/pain group. More patients in the skeletal group required any use of mobility aids (22.6% vs 3.5%, respectively; P=0.001). Six-Minute Walk test distances walked were similar between groups. SF-36v2 and HAQ-DI scores were similar between groups for physical component summary (n=238; mean [SD]: 40.2 [11.0] vs 43.6 [11.2]; P=0.056), mental component summary (n=238; mean [SD]: 43.6 [11.3] vs 43.8 [11.8]; P=0.902), and HAQ-DI (n=239; median [minimum, maximum]: 0.4 [0.0, 2.7] vs 0.3 [0.0, 2.1]; P=0.22).
Conclusion: Adults with HPP experience similar QoL impairment regardless of skeletal involvement.
Registration: https://clinicaltrials.gov/ct2/show/NCT02306720 and https://www.encepp.eu/encepp/viewResource.htm?id=47907, identifier NCT02306720; EUPAS13514.
Keywords: 6MWT; SF-36; alkaline phosphatase; fracture; health-related quality of life; mobility; pseudofracture; rare diseases.
Copyright © 2023 Dahir, Kishnani, Martos-Moreno, Linglart, Petryk, Rockman-Greenberg, Martel, Ozono, Högler and Seefried.
Conflict of interest statement
Authors AP and SM are employed by Alexion, AstraZeneca Rare Disease. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that this study received funding from Alexion, AstraZeneca Rare Disease. The funder had the following involvement in the study: conception and design of the Registry, collection of data, and statistical analyses, as described in the Author Contributions section.
Figures


References
-
- Rockman-Greenberg C. Hypophosphatasia. Pediatr Endocrinol Rev (2013) 10(suppl 2):380–8. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

