Vaccinating women previously treated for human papillomavirus-related cervical precancerous lesions is highly cost-effective in China
- PMID: 37051255
- PMCID: PMC10083292
- DOI: 10.3389/fimmu.2023.1119566
Vaccinating women previously treated for human papillomavirus-related cervical precancerous lesions is highly cost-effective in China
Abstract
Background: The 2021 Chinese Expert Consensus on the Clinical Application of the Human Papillomavirus (HPV) Vaccine recommended vaccination for women who previously received ablative or excisional treatment for high-grade squamous intraepithelial lesion (HSIL). This study evaluates the cost-effectiveness of HPV vaccination in women previously treated for cervical precancerous lesions.
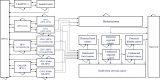
Methods: We used a Markov model to simulate the disease progression of both low- and high-risk HPV subtypes. We followed a cohort of 100,000 women aged 18-45 years who received treatment for cervical precancerous lesions for a lifetime (80 years). We used the Incremental Cost-Effectiveness Ratios (ICER) with a 5% discount rate to measure the cost-effectiveness of nine vaccination strategies, including a combination of HPV bivalent (HPV-2), quadrivalent (HPV-4) and nonavalent vaccine (HPV-9), each with three vaccination doses (one-, two- and three-dose). We conducted one-way sensitivity analysis and probabilistic sensitivity analysis. We followed the CHEERS 2022 guidelines.
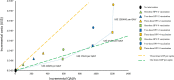
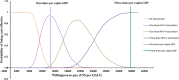
Results: Compared to the status quo, the nine vaccination strategies would result in $3.057-33.124 million incremental cost and 94-1,211 incremental quality-adjusted life-years (QALYs) in 100,000 women previously treated for cervical precancerous lesions. Three vaccination strategies were identified on the cost-effectiveness frontier. In particular, ICER for one-dose HPV-4 vaccination was US$10,025/QALY compared to the status quo (no vaccination); ICER for two-dose HPV-4 vaccination was US$17,641//QALY gained compared to one-dose HPV-4 vaccination; ICER for three-dose HPV-4 vaccination was US$27,785/QALY gained compared with two-dose HPV-4 vaccination. With a willingness-to-pay of three times gross domestic product per capita (US$37655), three-dose HPV-4 vaccination was the most cost-effective vaccination strategy compared with the lower-cost non-dominated strategy on the cost-effectiveness frontier. A probabilistic sensitivity analysis confirmed a 99.1% probability of being cost-effective. If the cost of the HPV-9 is reduced to 50% of the current price, three-dose HPV-9 vaccination would become the most cost-effective strategy.
Discussion: Three-dose HPV-4 vaccination is the most cost-effective vaccination strategy for women treated for precancerous cervical lesions in the Chinese setting.
Keywords: HPV vaccination; cervical precancerous lesions; health economics (cost-effectiveness analysis); healthcare provider; women.
Copyright © 2023 Zou, Liu, Liu, Wang, Zou and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Cost-effectiveness of human papillomavirus (HPV) vaccination in Tunisia: a modelling study.BMJ Open. 2024 Dec 5;14(12):e085462. doi: 10.1136/bmjopen-2024-085462. BMJ Open. 2024. PMID: 39638594 Free PMC article.
-
Incremental cost-effectiveness evaluation of vaccinating girls against cervical cancer pre- and post-sexual debut in Belgium.Vaccine. 2013 Aug 20;31(37):3962-71. doi: 10.1016/j.vaccine.2013.06.008. Epub 2013 Jun 15. Vaccine. 2013. PMID: 23777952
-
Prophylaxis of cervical cancer and related cervical disease: a review of the cost-effectiveness of vaccination against oncogenic HPV types.J Manag Care Pharm. 2010 Apr;16(3):217-30. doi: 10.18553/jmcp.2010.16.3.217. J Manag Care Pharm. 2010. PMID: 20331326 Free PMC article. Review.
-
Domestic HPV vaccine price and economic returns for cervical cancer prevention in China: a cost-effectiveness analysis.Lancet Glob Health. 2020 Oct;8(10):e1335-e1344. doi: 10.1016/S2214-109X(20)30277-1. Lancet Glob Health. 2020. PMID: 32971056
-
Should human papillomavirus vaccination target women over age 26, heterosexual men and men who have sex with men? A targeted literature review of cost-effectiveness.Hum Vaccin Immunother. 2018;14(12):3010-3018. doi: 10.1080/21645515.2018.1496878. Epub 2018 Sep 11. Hum Vaccin Immunother. 2018. PMID: 30024823 Free PMC article. Review.
Cited by
-
HPV vaccination strategy for 14-year-old females and economic returns for cervical cancer prevention in Wuxi City, China: a cost effectiveness analysis.Cost Eff Resour Alloc. 2024 Sep 5;22(1):64. doi: 10.1186/s12962-024-00574-9. Cost Eff Resour Alloc. 2024. PMID: 39237947 Free PMC article.
-
Examining the impact of sex-biased information on health behaviors: a study of HPV vaccination among male college students based on the extended theory of planned behavior.Front Public Health. 2025 May 9;13:1525547. doi: 10.3389/fpubh.2025.1525547. eCollection 2025. Front Public Health. 2025. PMID: 40416654 Free PMC article.
-
Opportunities and challenges for human papillomavirus vaccination in China.Hum Vaccin Immunother. 2024 Dec 31;20(1):2329450. doi: 10.1080/21645515.2024.2329450. Epub 2024 Apr 4. Hum Vaccin Immunother. 2024. PMID: 38575524 Free PMC article. Review.
-
Current Trends in Messenger RNA Technology for Cancer Therapeutics.Biomater Res. 2025 Apr 9;29:0178. doi: 10.34133/bmr.0178. eCollection 2025. Biomater Res. 2025. PMID: 40207255 Free PMC article. Review.
-
Cost-Effectiveness of 9-Valent HPV Vaccination for Patients Treated for High-Grade Cervical Intraepithelial Neoplasia in the UK.JAMA Netw Open. 2024 Oct 1;7(10):e2437703. doi: 10.1001/jamanetworkopen.2024.37703. JAMA Netw Open. 2024. PMID: 39365579 Free PMC article.
References
-
- Bruni L AG, Serrano B, Mena M, Collado JJ, Gómez D, Muñoz J, et al. . Human papillomavirus and related diseases in China, ICO/IARC information centre on HPV and cancer (HPV information centre). (2021). Available at:https://hpvcentre.net/statistics/reports/CHN.pdf?t=1652620911848.
-
- Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, Castellsagué X, et al. . Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol (2012) 13:89–99. doi: 10.1016/S1470-2045(11)70286-8 - DOI - PubMed

