Cell-specific occupancy dynamics between the pioneer-like factor Opa/ZIC and Ocelliless/OTX regulate early head development in embryos
- PMID: 37051467
- PMCID: PMC10083704
- DOI: 10.3389/fcell.2023.1126507
Cell-specific occupancy dynamics between the pioneer-like factor Opa/ZIC and Ocelliless/OTX regulate early head development in embryos
Abstract
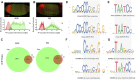
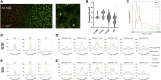
During development, embryonic patterning systems direct a set of initially uncommitted pluripotent cells to differentiate into a variety of cell types and tissues. A core network of transcription factors, such as Zelda/POU5F1, Odd-paired (Opa)/ZIC3 and Ocelliless (Oc)/OTX2, are conserved across animals. While Opa is essential for a second wave of zygotic activation after Zelda, it is unclear whether Opa drives head cell specification, in the Drosophila embryo. Our hypothesis is that Opa and Oc are interacting with distinct cis-regulatory regions for shaping cell fates in the embryonic head. Super-resolution microscopy and meta-analysis of single-cell RNAseq datasets show that opa's and oc's overlapping expression domains are dynamic in the head region, with both factors being simultaneously transcribed at the blastula stage. Additionally, analysis of single-embryo RNAseq data reveals a subgroup of Opa-bound genes to be Opa-independent in the cellularized embryo. Interrogation of these genes against Oc ChIPseq combined with in situ data, suggests that Opa is competing with Oc for the regulation of a subgroup of genes later in gastrulation. Specifically, we find that Oc binds to late, head-specific enhancers independently and activates them in a head-specific wave of zygotic transcription, suggesting distinct roles for Oc in the blastula and gastrula stages.
Keywords: Drosophila embryo; Oc/OTX; Opa/ZIC; embryonic head development; epigenetics.
Copyright © 2023 Fenelon, Gao, Borad, Abbasi, Pachter and Koromila.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Odd-paired is a pioneer-like factor that coordinates with Zelda to control gene expression in embryos.Elife. 2020 Jul 23;9:e59610. doi: 10.7554/eLife.59610. Elife. 2020. PMID: 32701060 Free PMC article.
-
Odd-Paired: The Drosophila Zic Gene.Adv Exp Med Biol. 2018;1046:41-58. doi: 10.1007/978-981-10-7311-3_3. Adv Exp Med Biol. 2018. PMID: 29442316 Review.
-
Zygotic pioneer factor activity of Odd-paired/Zic is necessary for late function of the Drosophila segmentation network.Elife. 2020 Apr 29;9:e53916. doi: 10.7554/eLife.53916. Elife. 2020. PMID: 32347792 Free PMC article.
-
The Zic family member, odd-paired, regulates the Drosophila BMP, decapentaplegic, during adult head development.Development. 2007 Apr;134(7):1301-10. doi: 10.1242/dev.02807. Epub 2007 Feb 28. Development. 2007. PMID: 17329368
-
From egg to gastrula: how the cell cycle is remodeled during the Drosophila mid-blastula transition.Annu Rev Genet. 2014;48:269-94. doi: 10.1146/annurev-genet-111212-133531. Epub 2014 Sep 5. Annu Rev Genet. 2014. PMID: 25195504 Free PMC article. Review.
Cited by
-
Su(H) Modulates Enhancer Transcriptional Bursting in Prelude to Gastrulation.Cells. 2024 Oct 24;13(21):1759. doi: 10.3390/cells13211759. Cells. 2024. PMID: 39513866 Free PMC article.
References
-
- Acampora D., Di Giovannantonio L. G., Di Salvio M., Mancuso P., Simeone A. (2009). Selective inactivation of Otx2 mRNA isoforms reveals isoform-specific requirement for visceral endoderm anteriorization and head morphogenesis and highlights cell diversity in the visceral endoderm. Mech. Dev. 126, 882–897. 10.1016/j.mod.2009.07.003 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

