Efficacy and Safety of Rifaximin in the Prevention of Recurrent Episodes of Hepatic Encephalopathy: A Systematic Review and Meta-analysis
- PMID: 37051626
- PMCID: PMC10441098
- DOI: 10.5152/tjg.2023.22575
Efficacy and Safety of Rifaximin in the Prevention of Recurrent Episodes of Hepatic Encephalopathy: A Systematic Review and Meta-analysis
Abstract
Background: Rifaximin is an oral antimicrobial drug with a broad-spectrum effect. It locally regulates the function and structure of intestinal bacteria and decreases intestinal endotoxemia. We aimed to investigate the preventive role of rifaximin in recurrent episodes of hepatic encephalopathy in cases with a history of hepatic diseases.
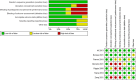
Methods: We searched PubMed, Scopus, and Web of Science for the relevant studies using the following search strategy: "(Rifaximin) OR (Xifaxan) AND (cirrhosis) OR (encephalopathy)." We assessed the risk of bias using Cochrane's risk of bias tool. We included the following outcomes: recurrence of hepatic encephalopathy, adverse events, mortality rate, and time to the first episode of hepatic encephalopathy from the time of randomization (days). We performed the analysis of homogeneous data under the fixed-effects model, while analysis of heterogeneous data was performed under the random-effects model.
Results: We analyzed data obtained from 999 patients from 7 included trials. The overall risk ratio proved that the rifaximin group was associated with a lower recurrence rate than the control group (risk ratio [RR] = 0.61[0.50, 0.73], P = .001). We found no significant variation in both groups regarding adverse events (RR = 1.08 [0.89, 1.32], P = .41), and mortality rates (RR = 0.98 [0.61, 1.57], P = .93). The overall risk of bias results was low.
Conclusion: The meta-analysis showed that in patients allocated to the rifaximin group, the incidence rate of hepatic encephalopathy was significantly lower when compared with those in the control group with no difference in both groups regarding adverse events and mortality rates.
Conflict of interest statement
Figures


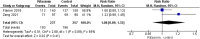


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
